J Res Clin Med. 12:32.
doi: 10.34172/jrcm.34629
Review Article
Effect of magnesium sulfate on post-operative pain: A systematic review and meta-analysis
Ata Mahmoodpoor Data curation, 1 
Kaveh Latifi Formal analysis, Writing – original draft, Writing – review & editing, 2 
Morteza Ghojazadeh Formal analysis, Methodology, Software, Validation, 3 
Mohammad Ebrahim Nikbakht Investigation, 4
Mohammad-Salar Hosseini Methodology, 4 
Tohid Sarfaraz Software, 1
Hassan Soleimanpour Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing, 5, * 
Author information:
1Anesthesiology Research Team, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Anesthesiology and Pain Medicine, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
3Research Center for Evidence Based Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
4Student Research Committee, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
5Road Traffic Injury Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Magnesium sulfate is a well-known analgesic, acting by antagonizing N-methyl-D-aspartate (NMDA) receptors in the central nervous system.
Methods:
A systematic search of databases was conducted, 2388 articles were identified and reviewed and 34 articles containing control and intervention groups were finally entered. Terms and keywords were selected based on PICO. The titles of obtained articles were first evaluated and repetitive titles were excluded. Then, the full texts of the remaining articles were studied, and those based on inclusion and exclusion criteria were evaluated. In addition, studies investigated in terms of the risk of bias (selection, performance, reporting, attrition, etc.), and content and studies that did not have the appropriate quality for the above reasons were excluded. EndNote X7 software used for managing resources, organizing studies, and identifying repetitive cases. The extracted information from the articles was analyzed by meta-analyzing. The dissimilarity between the studies was checked by Cochran’s Q test and I2 statistics.
Results:
Postoperative pain is one of the major concerns of anesthesiologists, and its poor management can be associated with adverse events. The main goals of this study are to examine the effect of magnesium sulfate consumption on the first-time analgesic request after surgery, the amount of analgesic consumption after surgery, the patient’s pain score, and patient satisfaction after surgery. According to the P value (0.93), it can be concluded that the amount of pain in the first 24 hours of the two groups is not different. It can be concluded that the amount of pain in the first hour of the two groups is different (P value=0.04). The P value (0.0007) shows that the amount of analgesic request in the first 24 hours of the two groups is different. Except for one study, this study showed that magnesium sulfate made a significant improvement in terms of patient satisfaction. Overall, the use of magnesium sulfate significantly prolonged the time to request analgesics.
Conclusion:
Since magnesium sulfate is a cost-effective drug, without major risk and a wide reliable serum range, the effect of this drug as an adjuvant should be further investigated.
Keywords: Analgesia, Magnesium sulfate, Pain, Post-operation, Surgery
Copyright and License Information
© 2024 The Authors.
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study was supported by Road Traffic Injury Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Introduction
Magnesium sulfate is a well-known and valuable analgesic, and its mechanism of analgesia is based on the antagonism of N-methyl-D-aspartate (NMDA) receptor in the central nervous system.1-4 These properties have encouraged people to research magnesium as an adjuvant agent for intra- and post-operative analgesia.5 Magnesium sulfate is used in diverse clinical scenarios, including tachyarrhythmias, myocardial ischemia, asthma and convulsions.4,5 In this systematic review, we intended to define the effectiveness of intravenous magnesium sulfate in post-surgical pain management, as postoperative pain is a complex physiological response to tissue damage, and its management is one of the main priorities of anesthesiologists.6 The use of magnesium has become common for years to manage and reduce the pain of patients after surgery. For the first time in a study in 1991, magnesium as an adjuvant analgesic, was used to reduce postoperative pain.7 The results of many previous clinical researches have shown that infusion of magnesium sulfate during general anesthesia reduces postoperative pain and the need for opioid and non-opioid analgesics after surgery.1 Due to the significant advantages of magnesium sulfate over other available treatments in terms of low drug interference, cost-effectiveness, minor side effects and availability, proving the effectiveness of magnesium sulfate in reducing pain after surgery will be an important step toward improving patient conditions, increasing patient satisfaction and reducing hospital stays and costs. However, There is no comprehensive and systematic review in this regard, which necessitates conducting such a study to resolve these scientific ambiguities. Therefore, the main goal of this study is to investigate the effect of magnesium sulfate on postoperative pain management and reduce the amount and dosage of other common drugs especially opioids and their possible side effects, which are always a concern of anesthesiologists.
Materials and Methods
Literature search
Embase, PubMed, Ovid, Scopus, ProQuest, Web of Knowledge, and the Cochrane Libraries and Google Scholar databases were searched to identify all articles until the end of 2022 regarding the analgesic effects of magnesium sulfate. For better identification and coverage, other sources, including grey literature and articles presented in congress, were also searched. Terms and keywords were selected based on PICO, which is listed below in Table 1.
Table 1.
PICO (Population, Intervention, Comparison, Outcome) components
|
|
Keywords
|
| P |
Post-operation, post-surgery, perioperative, peri surgery, post-laparoscopy, post-laparotomy, operation, surgery, anesthesia, general anesthesia, spinal anesthesia |
| I |
Intravenous, magnesium, magnesium sulfate, MgSO4, Mg |
| C |
Control, normal saline, placebo, N/S |
| O |
Pain management, pain relief, analgesic, analgesia, pain control, post-operation effects, side effects, pain, analgesia |
Selection of studies and data extraction
The articles obtained from the search were first evaluated in terms of titles, and the repetitive titles were excluded. The abstracts of all the remaining studies and studies unrelated to the research objectives were excluded from the study, then the full text of the remaining articles were reviewed based on the inclusion and exclusion criteria. Studies with irrelevant subjects and low quality were also excluded. In addition, studies investigated in terms of the risk of bias (selection, performance, reporting, attrition, etc), and content and studies that did not have the appropriate quality for the above reasons were excluded. EndNote X7 software used for managing resources, organizing studies and identifying repetitive cases. When differences between observations by two authors in cases of disagreement with eligibility were not resolved discrepancy, the opinion of the third author (M.G.) was accepted for problem solving.
Inclusion and exclusion criteria
Inclusion criteria for the studies were as following: (1) RCTs in which the intervention group received magnesium sulfate intravenously along with the control group that received normal saline. (2) Studies that have investigated patients in terms of postoperative pain and the amount of analgesic consumption. Exclusion criteria for the studies were as following: (1) Studies conducted on animal groups. (2) Studies that did not have a control group (3) Studies that did not use magnesium sulfate intravenously. (4) Articles that did not have the required quality.
Statistical analysis
The information extracted from the articles was finally analyzed by meta-analyzing. Dissimilarity between the studies was checked by Cochran’s Q test and I2 statistics, which express the percentage of changes between the studies. If the statistical values of I2 were less than a fixed effects model was used, and if it was more than 50% search engine, the random effects model was used to calculate the overall size. CMA software was used for statistical analysis, and a P value less than 0.05 was considered significant.
Results
Search results and study characteristics
A systematic search of databases was conducted, and 2388 articles were identified, 287 of them were removed by the software as they were repetitive, and 2101 articles were reviewed in terms of title and abstract. Thereafter, 1871 of the articles, for various reasons such as repetitiveness, non-RCT study type, or irrelevance, were excluded and a number of 230 articles related to the study topic were reviewed in order to find the full text of them, but from which the full texts of four articles were not found. The remnant 226 articles were subjected to a more detailed full text review to examine the inclusion and exclusion criteria, which resulted in 34 articles to be evaluated in our study (Figure 1).
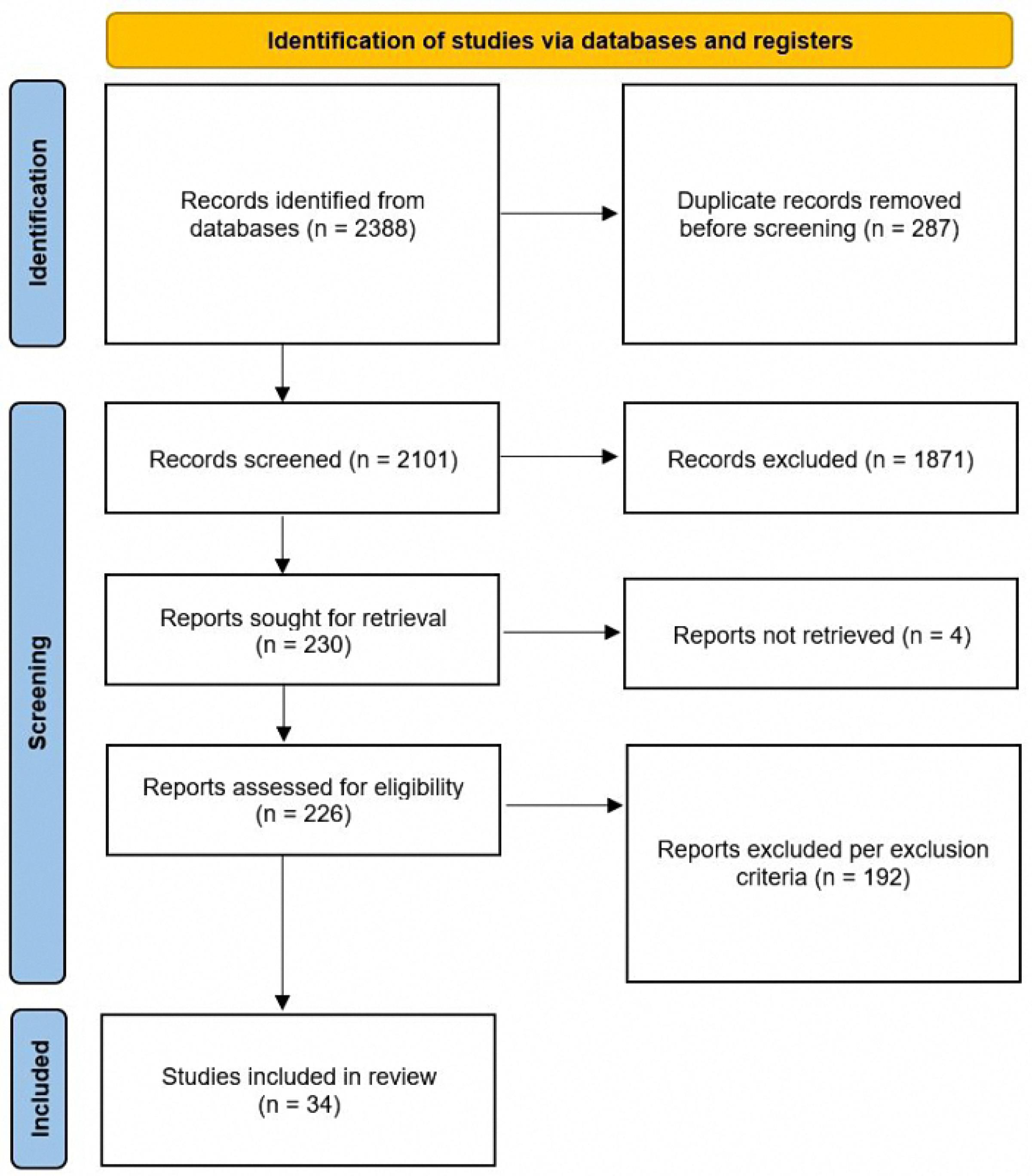
Figure 1.
Flowchart of the study
.
Flowchart of the study
Meta-analysis
34 studies, containing control and intervention groups, were finally entered into the meta-analysis (Table 2).8-41 To assess pain in these studies, two criteria were used, which are as follows: the VAS criterion (82% of studies) and the NRS criterion (18% of studies). According to the P value ( > 0.0001) of the homogeneity test, as well as the value of I2, which is a very high value (92.6) and the value of the variance of inhomogeneity between studies (tau), it is concluded that there is inhomogeneity between studies and the random effect method should be used (0.0743 [-1.5269; 1.6755] 0.09 0.9276). According to the P value (0.93), it can be concluded that the amount of pain in the first 24 hours of the two groups is not different. According to the first output, part of which is shown in the diagram above (the lack of homogeneity in the study), the part related to the random effect is of interest, and the result is that the amount of pain in the two groups is generally not different because the confidence interval (1.68 and -1.53) includes zero (Figures 2 and 3). According to the P value ( < 0.0001) of the homogeneity test, as well as the value of I2, which is a very high value (93.2), as well as the value of the variance in homogeneity between studies (tau) and its confidence interval, which does not include zero; It is concluded that there is a lack of homogeneity between the studies and the random effect method should be used (-1.9117 [-3.7156; -0.1077] -2.08 0.0378). According to the P value (0.04), it can be concluded that the amount of pain in the first 1 hour of the two groups is different. According to the first output, part of which is shown in the diagram above (the lack of homogeneity in the study), the part related to the random effect is of interest, and the result is that the amount of pain in the first hour of the two groups is different. Because the confidence interval (-3.72, -0.11) does not include zero, the amount of pain in the first hour has decreased for the case group (Figures 4 and 5). There was a significant difference between the control and intervention groups regarding analgesic consumption. Only 20% or seven studies did not show a significant decrease in the amount of analgesic consumption. According to the P value ( < 0.0001) of the homogeneity test, as well as the value of I2, which is a very high value (93.6%) and the value of the variance of inhomogeneity between studies (tau), it is concluded that there is inhomogeneity between studies and the random effect method should be used (-1.5377 [-2.4217; -0.6537] -3.41 0.0007). According to the P value (0.0007), it is concluded that the amount of analgesics in the first 24 hours of the two groups are different. According to the first output, a part of which is shown in the diagram above (lack of homogeneity in the study), the part related to the random effect is of interest and the result is that the amount of analgesic consumption in the 24 hours is different between the two groups because the confidence interval (4.78 and 3.25) does not include zero. It seems that the amount of analgesic consumption in the next 24 hours has decreased for the case group (Figures 6 and 7). 80% of the surgeries performed were under general anesthesia using a combination of fentanyl and propofol and only 20% of studies that used spinal anesthesia with bupivacaine, entered the study (seven studies). 74% of general anesthesias, were induced with propofol and the rest were induced with sodium thiopental. Fentanyl was the most prevalent opioid used during surgery and other opioids used were sufentanil,8 remifentanil,9-15 alfentanil16 and pethidine.17 Meanwhile, remifentanil has been used in seven studies and has been used abundantly. In 42% of studies that used spinal anesthesia, there was no significant difference in the pain score, which was not much different from the general study population. It seems that the type of anesthesia does not make a meaningful difference to the effect of magnesium sulfate (Tables 3 and 4). The most used drug was morphine sulfate, which included 53% of the studies. Only in one study, diclofenac17 was used as the main analgesic. Only 20% or seven studies did not show a significant decrease in the amount of analgesic consumption. Another aim of this study was to investigate the patients’ satisfaction between the two groups. Only four of the studies have reviewed patient satisfaction based on five score criteria. In only one study, patient satisfaction between two of the groups did not show a significant difference.18 Other studies showed that magnesium sulfate made a significant improvement in terms of patient satisfaction.13,19,20 The last goal of this research, which has been investigated by seven studies, was to evaluate the first-time an analgesic request. Among these seven studies, almost half of them did not show any significant difference between the two groups (42%), and in four studies, the use of magnesium sulfate significantly prolonged the time to request analgesics.20-23 The studies included in this meta-analysis have examined pain after various surgeries, which is as follows: below surgery is umbilical surgeries 45%, neurosurgery and spine surgery 11%, orthopedic surgeries 17%, surgeries above the umbilicus 21% and one study investigated VATS surgery 52%. Seven studies evaluated laparoscopic surgery, and the remaining surgeries (80%) were open. There is not any difference in terms of the relationship between the type of surgery and the amount of pain and the total amount of analgesic consumption (Table 3). By using Fisher’s exact test (P value = 0.28 and P value = 0.3), there were no relationship between the type of operation and the amount of analgesics and the amount of pain, respectively. The reviewed studies have used different doses and regimens of magnesium sulfate, which are as follows; just bolus (30%), first bolus and then infusion during surgery (65%), and only two studies received infusion without bolus.24,25 The lowest bolus dose used was 5 mg/kg26 and in another study, 8 mg/kg/hr was in the form of infusion.24 The most common regimen that has been used was 50 mg/kg bolus followed by infusion at a rate of 15 mg/kg/h (6 studies). In none of the studies, the toxic levels and side effects of magnesium were observed. Serum magnesium levels have been investigated (which is serum magnesium level before surgery and immediately after surgery) in 41% of studies. In most of the studies, serum magnesium levels between the intervention and control groups immediately after surgery, showed a significant increase in the intervention group with an exception٬27 in which magnesium sulfate was given in the form of a bolus of 50 kg/mg, while in other studies the regimen was a bolus followed by an infusion. None of the included studies investigated cerebrospinal fluid (CSF) magnesium levels. In order to assess the risk of bias in the studies, the checklist of JBI Institute was used, which is mentioned in Table 5.
Table 2.
Included studies
|
First Author
|
Year
|
Country
|
Case
|
Control
|
PSC1sth
|
PSCSE1sth
|
PSG11sth
|
PSG1SE1sth
|
| Tauzin-Fin P8 |
2006 |
France |
15 |
15 |
|
|
|
|
| Sousa AM9 |
2016 |
Brazil |
18 |
18 |
2 |
2.5 |
1 |
0.25 |
| Olgun B10 |
2012 |
Turkey |
30 |
30 |
6.4 |
2.1 |
4.3 |
2.1 |
| Ryu JH11 |
2008 |
South Korea |
25 |
25 |
|
|
|
|
| Asadollah S12 |
2015 |
Iran |
15 |
15 |
|
|
|
|
| Wilder-Smith CH13 |
1997 |
Switzerland |
13 |
11 |
|
|
|
|
| Kiran S14 |
2011 |
India |
50 |
50 |
|
|
|
|
| Tsaousi G15 |
2020 |
Greece |
35 |
36 |
|
|
|
|
| El Mourad MB16 |
2019 |
Egypt |
40 |
40 |
|
|
|
|
| Samir EA17 |
2013 |
Egypt |
25 |
25 |
|
|
|
|
| KUMAR M18 |
2013 |
India |
30 |
30 |
|
|
|
|
| Haryalchi K19 |
2017 |
Iran |
20 |
20 |
|
|
|
|
| Kayalha H20 |
2019 |
Iran |
30 |
30 |
|
|
|
|
| Taheri A21 |
2015 |
Iran |
20 |
20 |
|
|
|
|
| Ghaffaripour S22 |
2016 |
Iran |
19 |
20 |
|
|
|
|
| Bhatia A23 |
2004 |
India |
25 |
25 |
|
|
|
|
| MENTES O.4 |
2008 |
Turkey |
41 |
42 |
|
|
|
|
| Koinig H25 |
1998 |
Austria |
23 |
23 |
2.1 |
1 |
1.8 |
1.1 |
| Mahajan C26 |
2019 |
India |
15 |
15 |
2.8 |
0.4 |
1.8 |
0.4 |
| Bačak Kocman I.B.27 |
2013 |
Croatia |
20 |
20 |
5.2 |
2 |
4.7 |
1.7 |
| Menshawi MA28 |
2022 |
Egypt |
30 |
30 |
|
|
|
|
| Benevides ML29 |
2021 |
Brazil |
45 |
41 |
|
|
|
|
| Demiroglu M30 |
2016 |
Turkey |
25 |
25 |
4.5 |
0.1 |
3.4 |
0.1 |
| Shin HJ31 |
2016 |
South Korea |
22 |
22 |
|
|
|
|
| Moon S32 |
2020 |
South Korea |
31 |
30 |
|
|
|
|
| Mavrommati PD33 |
2004 |
Greece |
21 |
21 |
|
|
|
|
| Kizilcik N34 |
2018 |
Turkey |
40 |
40 |
|
|
|
|
| Kim HY35 |
2021 |
South Korea |
26 |
26 |
|
|
|
|
| Kaya S36 |
2009 |
Turkey |
20 |
20 |
|
|
|
|
| Jarahzadeh MH37 |
2016 |
Iran |
30 |
30 |
8.6 |
0.67 |
8.1 |
0.99 |
| Hwang JY38 |
2010 |
South Korea |
20 |
20 |
|
|
|
|
| Ebrahimy Dehkordy M39 |
2020 |
Iran |
40 |
40 |
1.8 |
2 |
1.6 |
1.8 |
| Dabbagh A40 |
2009 |
Iran |
30 |
30 |
2.5 |
0.7 |
1.1 |
0.3 |
| Ayoglu H41 |
2005 |
Turkey |
20 |
20 |
3.56 |
0.7 |
2.5 |
0.5 |
PSC1sth: Pain score after 1st hour - control group (mean), PSCSE1sth: Pain score after 1st hour - control group (SD), PSG11sth: Pain score after 1st hour - intervention group (mean), PSG1SE1sth: Pain Score after 1st hour - intervention group (SD)
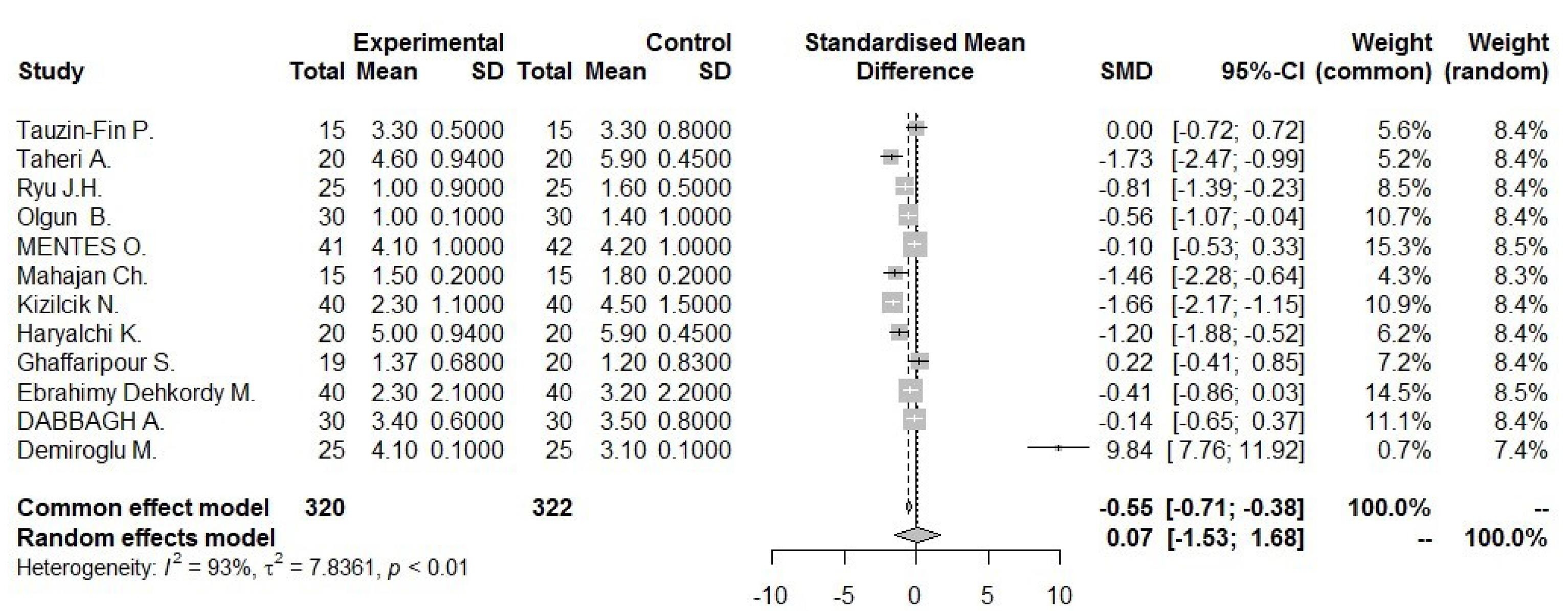
Figure 2.
Forest plot for pain score 24 hour
.
Forest plot for pain score 24 hour
Figure 3.
Funnel plot for pain score 24 hour
.
Funnel plot for pain score 24 hour
Figure 4.
Forest plot for pain score 1 hour
.
Forest plot for pain score 1 hour
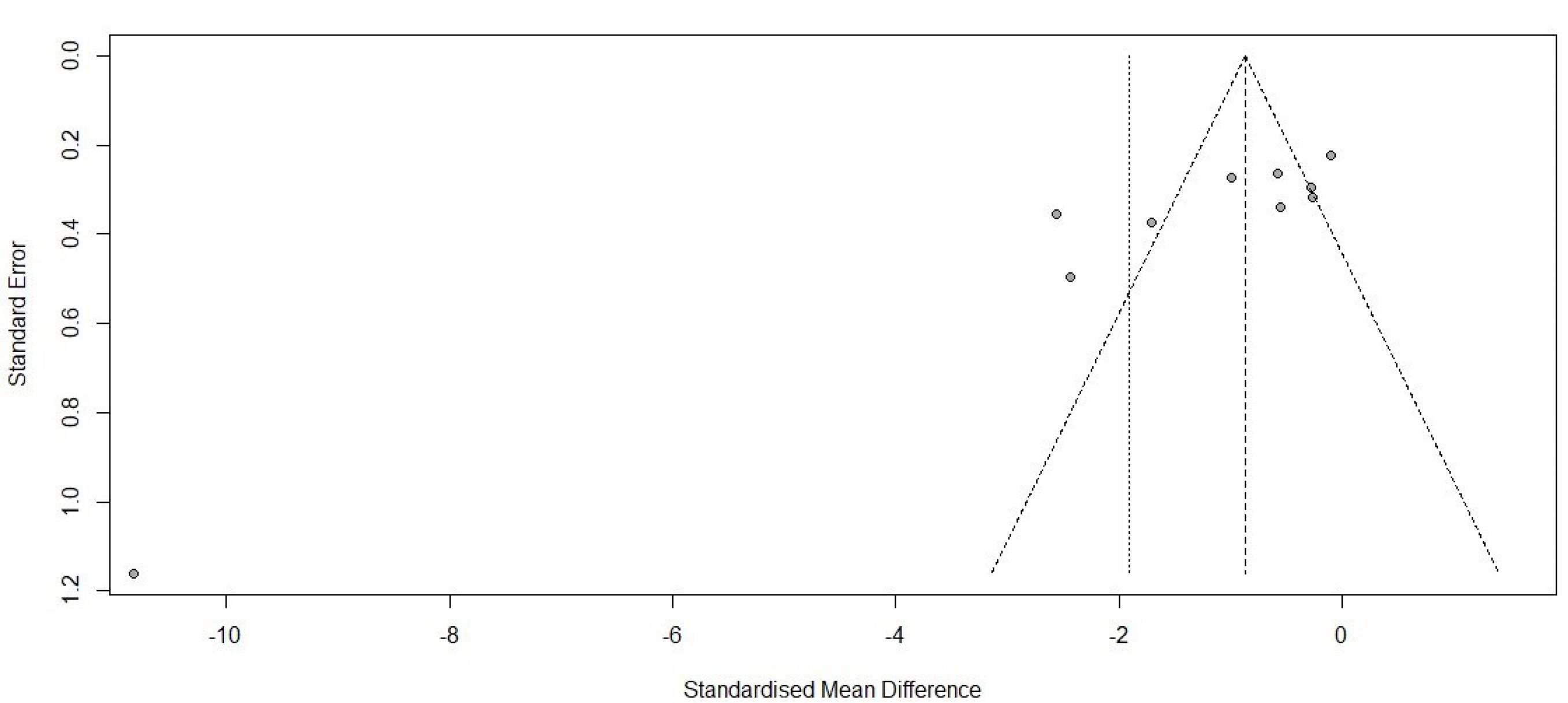
Figure 5.
Funnel plot for pain score 1 hour
.
Funnel plot for pain score 1 hour
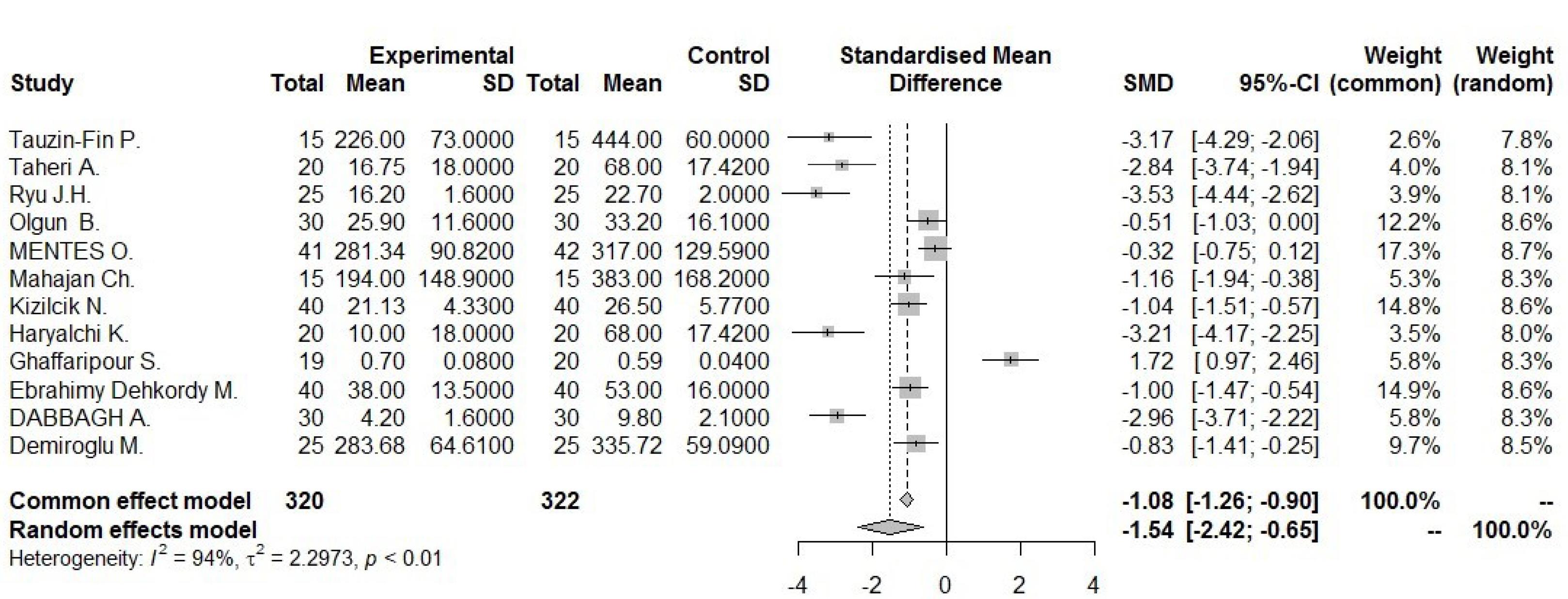
Figure 6.
Forest Plot for amount of analgesic
.
Forest Plot for amount of analgesic
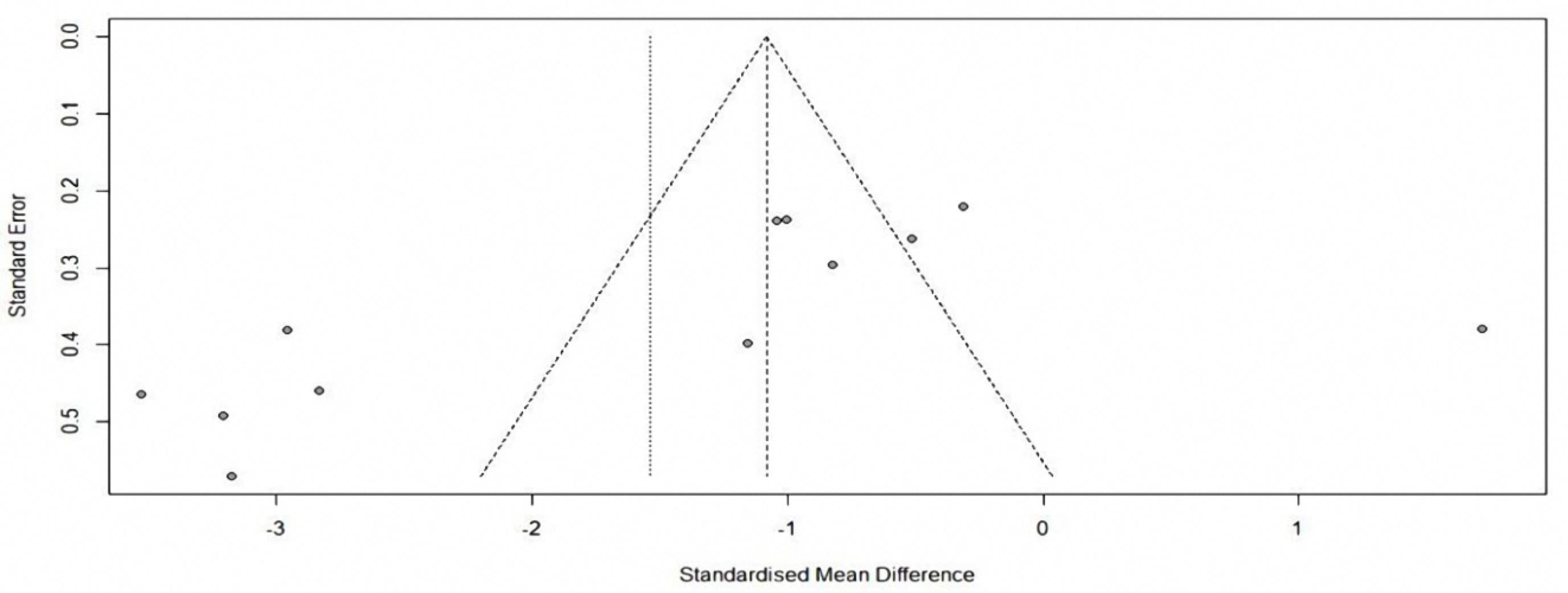
Figure 7.
Funnel Plot for amount of analgesic
.
Funnel Plot for amount of analgesic
Table 3.
Frequency of the used analgesics
|
Analgesic drugs
|
ANALGESCSE
|
ANALGESC24h
|
ANALGESG1SE
|
ANALGESG124h
|
PSCSE
|
PSC24h
|
| Morphine |
|
46 |
|
41.5 |
|
1 |
| Morphine |
4.6 |
14.5 |
3.37 |
5.33 |
|
2 |
| Tramadol |
60 |
444 |
73 |
226 |
0.8 |
3.3 |
| Pethidine |
17.42 |
68 |
18 |
16.75 |
0.45 |
5.9 |
| Morphine |
6.3 |
12 |
6.2 |
5.7 |
0.4 |
1.4 |
| Fentanyl |
|
|
|
|
1.1 |
2.9 |
| Morphine |
2 |
22.7 |
1.6 |
16.2 |
0.5 |
1.6 |
| Morphine |
16.1 |
33.2 |
11.6 |
25.9 |
1 |
1.4 |
| Fentanyl |
10 |
330 |
10 |
233 |
|
3 |
| Tramadol |
129.59 |
317 |
90.82 |
281.34 |
1 |
4.2 |
| Nalbuphine |
7.05 |
49.06 |
5.13 |
40.32 |
|
3 |
| Fentanyl |
|
|
|
|
|
|
| Fentanyl |
168.2 |
383 |
148.9 |
194 |
0.2 |
1.8 |
| Morphine |
2.68 |
7.13 |
1.25 |
3.99 |
|
2.12 |
| Fentanyl |
|
|
|
|
|
|
| Metamizol |
|
140 |
|
115 |
1.6 |
1.3 |
| Morphine |
5.77 |
26.5 |
4.33 |
21.13 |
1.5 |
4.5 |
| Diclofenac |
|
63.8 |
|
26.1 |
0.46 |
1.3 |
| Morphine |
|
|
|
|
0.8 |
1.8 |
| Morphine |
|
25 |
|
20 |
|
5 |
| Morphine |
7.3 |
36.7 |
10.2 |
30.2 |
|
|
| Morphine |
|
|
|
|
|
|
| Morphine |
12.5 |
50 |
11 |
28.3 |
|
3.8 |
| Pethidine |
17.42 |
68 |
18 |
10 |
0.45 |
5.9 |
| Morphine |
0.04 |
0.59 |
0.08 |
0.7 |
0.83 |
1.2 |
| Morphine |
16 |
53 |
13.5 |
38 |
2.2 |
3.2 |
| Morphine |
2.1 |
9.8 |
1.6 |
4.2 |
0.8 |
3.5 |
| Morphine |
0.05 |
0.23 |
0.05 |
0.28 |
|
3.3 |
| Tramadol |
67.8 |
29.2 |
36.6 |
15.5 |
|
2 |
| Morphine |
|
|
|
|
|
|
| Pethidine |
|
85.6 |
|
42 |
|
3.1 |
| Meperidine |
22.9 |
164 |
24.49 |
118 |
|
2 |
| Morphine |
2.1 |
9.1 |
3.1 |
7.4 |
|
3.6 |
| Tramadol |
59.09 |
335.72 |
64.61 |
283.68 |
0.1 |
3.1 |
ANALGESCSE: 24h post-operative analgesic consumption - control group (SD), ANALGESC24h: 24h post-operative analgesic consumption - control group (mean), ANALGESG1SE: 24h post-operative analgesic consumption - intervention group (SD), ANALGESG124h: 24h post-operative analgesic consumption - intervention group (mean), PSCE: Pain score after 24 hours - control group (SD), PSC24h: Pain score after 24 hours - control group (mean)
Table 4.
Type of surgery and the amount of pain and the total amount of analgesic consumption
|
TOS
|
MixedPS
|
MixedANALGES
|
| Below umbilical surgery |
1 |
41.5 |
| Neurosurgery |
1 |
5.33 |
| Below umbilical surgery |
3.3 |
226 |
| Below umbilical surgery |
4.6 |
16.75 |
| Below umbilical surgery |
|
5.7 |
| Orthopedic surgery |
1.9 |
|
| Below umbilical surgery |
1 |
16.2 |
| Upper umbilical surgery |
1 |
25.9 |
| Below umbilical surgery |
3 |
233 |
| Upper umbilical surgery |
4.1 |
281.34 |
| VATS |
2.5 |
40.32 |
| Below umbilical surgery |
|
|
| Neurosurgery |
1.5 |
194 |
| Below umbilical surgery |
2.05 |
3.99 |
| Orthopedic surgery |
|
|
| Upper umbilical surgery |
1.6 |
115 |
| Upper umbilical surgery |
2.3 |
21.13 |
| Below umbilical surgery |
0.78 |
26.1 |
| Below umbilical surgery |
1.6 |
|
| Orthopedic surgery |
4 |
20 |
| Below umbilical surgery |
|
30.2 |
| Below umbilical surgery |
|
|
| Orthopedic surgery |
2 |
28.3 |
| Below umbilical surgery |
5 |
10 |
| Neurosurgery |
1.37 |
0.7 |
| Neurosurgery |
2.3 |
38 |
| Orthopedic surgery |
3.4 |
4.2 |
| Upper umbilical surgery |
3.5 |
0.28 |
| Below umbilical surgery |
3 |
15.5 |
| Upper umbilical surgery |
|
|
| Below umbilical surgery |
2.4 |
42 |
| Orthopedic surgery |
2 |
118 |
| Upper umbilical surgery |
4 |
7.4 |
| Neurosurgery |
4.1 |
283.68 |
| Below umbilical surgery |
1 |
46 |
| Neurosurgery |
2 |
14.5 |
| Below umbilical surgery |
3.3 |
444 |
| Below umbilical surgery |
5.9 |
68 |
| Below umbilical surgery |
1.4 |
12 |
| Orthopedic surgery |
2.9 |
|
| Below umbilical surgery |
1.6 |
22.7 |
| Upper umbilical surgery |
1.4 |
33.2 |
| Below umbilical surgery |
3 |
330 |
| Upper umbilical surgery |
4.2 |
317 |
| VATS |
3 |
49.06 |
| Below umbilical surgery |
|
|
| Neurosurgery |
1.8 |
383 |
| Below umbilical surgery |
2.12 |
7.13 |
| Orthopedic surgery |
|
|
| Upper umbilical surgery |
1.3 |
140 |
| Upper umbilical surgery |
4.5 |
26.5 |
| Below umbilical surgery |
1.3 |
63.8 |
| Below umbilical surgery |
1.8 |
|
| Orthopedic surgery |
5 |
25 |
| Below umbilical surgery |
|
36.7 |
| Below umbilical surgery |
|
|
| Orthopedic surgery |
3.8 |
50 |
| Below umbilical surgery |
5.9 |
68 |
| Neurosurgery |
1.2 |
0.59 |
| Neurosurgery |
3.2 |
53 |
| Orthopedic surgery |
3.5 |
9.8 |
| Upper umbilical surgery |
3.3 |
0.23 |
| Below umbilical surgery |
2 |
29.2 |
| Upper umbilical surgery |
|
|
| Below umbilical surgery |
3.1 |
85.6 |
| Orthopedic surgery |
2 |
164 |
| Upper umbilical surgery |
3.6 |
9.1 |
| Neurosurgery |
3.1 |
335.72 |
TOS: Type of Surgery, MixedPS: Mixed Patient Score, MixedANLGES: Mixed Analgesic Consumption, VATS: Video-Assisted Thoracoscopic Surgery
Table 5.
Checklist of JBI Institute for assessing the risk of bias in the studies
|
Author
|
Year
|
Q1
|
Q2
|
Q3
|
Q4
|
Q5
|
Q6
|
Q7
|
Q8
|
Q9
|
Q10
|
Q11
|
Q12
|
Q13
|
| Tauzin-Fin P8 |
2006 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Sousa AM9 |
2016 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
| Olgun B10 |
2012 |
Y |
U |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Ryu JH11 |
2008 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Asadollah S12 |
2015 |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Wilder-Smith CH13 |
1997 |
N |
U |
Y |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
| Kiran S14 |
2011 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Tsaousi G15 |
2020 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
| El Mourad MB16 |
2019 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Samir EA17 |
2013 |
U |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| KUMAR M18 |
2013 |
Y |
U |
Y |
Y |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Haryalchi K19 |
2017 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Kayalha H20 |
2019 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
| Taheri A21 |
2015 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
| Ghaffaripour S22 |
2016 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Bhatia A23 |
2004 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| MENTES O.4 |
2008 |
U |
U |
Y |
Y |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Koinig H25 |
1998 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
| Mahajan C26 |
2019 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
| Bačak Kocman I.B.27 |
2013 |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Menshawi MA28 |
2022 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
| Benevides ML29 |
2021 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
| Demiroglu M30 |
2016 |
U |
U |
Y |
Y |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Shin HJ31 |
2016 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Moon S32 |
2020 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
| Mavrommati PD33 |
2004 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
| Kizilcik N34 |
2018 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
| Kim HY35 |
2021 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Y |
Y |
| Kaya S36 |
2009 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
| Jarahzadeh MH37 |
2016 |
U |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
| Hwang JY38 |
2010 |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Ebrahimy Dehkordy M39 |
2020 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Dabbagh A40 |
2009 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Ayoglu H41 |
2005 |
Y |
U |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
Y (yellow): Yes, N (orange): No, U (green): Unclear
Discussion
The main drawn conclusion of this study and the review of 34 studies is that receiving intravenous magnesium sulfate before and during surgery can lead to two positive effects: reduction of the overall amount of analgesic consumption after surgery and prolongation of the first-time to request analgesics. But the effect of receiving systemic magnesium sulfate on postoperative pain still remains controversial and debatable. Comparing with previous systematic reviews and meta-analyses, it can be claimed that the current study is a new study where all types of surgery have been included with no restrictions. Intravenous administration of magnesium sulfate has obviously improved pain in a number of studies,15,19,28 but this positive effect is not visible in all studies.29,30 None study showed a worsening of pain intensity with magnesium sulfate intake. The most obvious effect of magnesium sulfate can be considered as reducing the total amount of consumption of analgesics after surgery in a way that only seven of the 34 studies did not show this significant reduction, and over 80% of studies have shown a significant reduction. The analgesics used were morphine, tramadol,30,31 pethidine٬15,25 diclofenac,17 fentanyl,32,33 meperidine٬22 metamizol,34 nalbufin,35 The highest frequency was related to morphine, with 53% prevalence. General anesthesia has been used in most studies, but the relationship between the type of anesthesia and the amount of analgesic consumption and pain intensity was not found. There was not any correlation between the type of anesthetics used and the study results. Regarding the first-time request for analgesics, which has been discussed in four of seven studies, a significant prolongation was observed.29 Regarding pain intensity after surgery, 18 studies showed a decrease in pain intensity in the magnesium sulfate group and among those, seven studies have reduced pain intensity with a P value < 0.001. This discrepancy can point out that each person has a different perception of pain and is influenced by different factors36 such as gender, psychological issues, genetics and even personality. Most studies have used the regimen of bolus and then infusion during surgery, while some studies have used bolus dose alone27,30 or even infusion alone regimens.24,25 The most commonly used bolus regimen was between 30-50 mg/kg, and the infusion rate in most studies was between 8-15 mg/kg/h. Benevides et al. in 2021 used the most common regimen with a bolus dose of 50 mg/kg and then an infusion rate of 15 mg/kg/h, which did not demonstrate a positive finding of reducing the amount of analgesics used and the pain intensity.37 Kayalha et al20 in 2019 used the lowest dose of magnesium sulfate, 5 mg/kg and the results have revealed a significant reduction in the analgesic consumption amount and intensity of pain. A study investigating different low doses of magnesium sulfate was conducted in Croatia in 2013 on candidates for laparoscopic cholecystectomy.34 Two groups received bolus regimens with doses of 5-7.5 mg/kg. In the conclusion, only the intensity of pain in the early hours after surgery showed a reduction.34 In another study, done on 80 people in Turkey in 2016,38 one group received a bolus magnesium sulfate dose of 40 mg/kg and the other two groups received a 10-20 mg/kg/h infusion after receiving the bolus dose. Ultimately, this study stated that bolus with infusion rate of 10 mg/kg/h was the best regimen.38 The overall rate of patient satisfaction has been investigated in five studies. In four studies, the rate of patient satisfaction was significantly higher after taking magnesium sulfate. In the included studies, surgeon satisfaction has not been investigated. In 14 studies, serum levels of magnesium were investigated before surgery and immediately after surgery and only one of these studies did not show a significant increase in serum magnesium levels in the intervention group.30 None studies investigated CSF magnesium levels. Hypermagnesemia is a rare event that occurs in clinical practice and serum levels more than 2.2 mg/dL can reduce blood pressure, reduce tendon reflexes and dizziness.39 The results of a systematic review conducted in 2018, on 11 clinical trial studies, in orthopedic surgeries, are consistent with this study. The most outstanding result, the reduction of the total amount of analgesics and the pain intensity of patients, was challenging.40
Three of the included studies, that have evaluated non-intravenous magnesium sulfate, showed promising results.21,22,41 A systematic review has not been performed on this issue, so that doubles its importance. Finally, the topic of pain and pain control will continue to be a challenging topic in medical research.42,43
Limitations
First, this study covered a wide range of surgeries with different lengths of surgery and anesthesia, different types of anesthesia used and different doses of magnesium sulfate; the characteristics of the patient population from different studies are also not homogeneous. Secondly, most of the included studies examined a small sample size so that the smallest sample size was 24 people and the largest sample size was 120 people. Due to the small statistical population, the results may have confronted biases.
Conclusion
In general, most of the studies included in this Meta analysis have reported a positive effect of magnesium sulfate before and during surgery, on pain management especially in the one postoperative hour and reducing the amount of analgesic consumption especially the opioids and their related complications, although some studies have revealed binary results. Since magnesium sulfate is a cost-effective drug, without major risks and a wide reliable serum range, the effect of this drug as an adjuvant should be further investigated. Clinical trials, with a larger statistical population, are better to be recruited to reduce the random error in this matter.
Acknowledgments
We would like to appreciate of the cooperation of Clinical Research Development Unit, Imam Reza General Hospital, Tabriz, Iran in conducting of this research. This research was extracted from the thesis for a M.D thesis by Mohammad Ebrahim Nikbakht entitled, “Effect of Magnesium Sulfate on post-operative pain: A systematic review”. It is registered at Tabriz University of Medical Sciences (No: 67280).
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no conflict of interest.
Ethical Approval
This study was approved by the Research Ethics Committee with No. IR.TBZMED.REC.1400.553 (2021-09-13).
References
- Soleimanpour H, Imani F, Dolati S, Soleimanpour M, Shahsavarinia K. Management of pain using magnesium sulphate: a narrative review. Postgrad Med 2022; 134(3):260-6. doi: 10.1080/00325481.2022.2035092 [Crossref] [ Google Scholar]
- Tramer MR, Schneider J, Marti RA, Rifat K. Role of magnesium sulfate in postoperative analgesia. Anesthesiology 1996; 84(2):340-7. doi: 10.1097/00000542-199602000-00011 [Crossref] [ Google Scholar]
- Morteza Bagi H, Ahmadi S, Tarighat F, Rahbarghazi R, Soleimanpour H. Interplay between exosomes and autophagy machinery in pain management: state of the art. Neurobiol Pain 2022; 12:100095. doi: 10.1016/j.ynpai.2022.100095 [Crossref] [ Google Scholar]
- Ascher P, Nowak L. Electrophysiological studies of NMDA receptors. Trends Neurosci 1987; 10(7):284-8. doi: 10.1016/0166-2236(87)90174-3 [Crossref] [ Google Scholar]
- Soleimanpour H, Aghamohammadi D, Ghaffarzad A, Ej Golzari S, Safari S, Soleimanpour M. Novel treatment in refractory tic douloureux. J Clin Anesth 2014; 26(6):495-6. doi: 10.1016/j.jclinane.2014.03.011 [Crossref] [ Google Scholar]
- Forkin KT, Nemergut EC. Miller’s anesthesia. Anesthesiology 2016; 124(4):977-8. doi: 10.1097/aln.0000000000001020 [Crossref] [ Google Scholar]
- Frassanito L, Messina A, Vergari A, Colombo D, Chierichini A, Della Corte F. Intravenous infusion of magnesium sulfate and postoperative analgesia in total knee arthroplasty. Minerva Anestesiol 2015; 81(11):1184-91. [ Google Scholar]
- Tauzin-Fin P, Sesay M, Delort-Laval S, Krol-Houdek MC, Maurette P. Intravenous magnesium sulphate decreases postoperative tramadol requirement after radical prostatectomy. Eur J Anaesthesiol 2006; 23(12):1055-9. doi: 10.1017/s0265021506001062 [Crossref] [ Google Scholar]
- Sousa AM, Rosado GM, de Sá Neto J, Guimarães GM, Ashmawi HA. Magnesium sulfate improves postoperative analgesia in laparoscopic gynecologic surgeries: a double-blind randomized controlled trial. J Clin Anesth 2016; 34:379-84. doi: 10.1016/j.jclinane.2016.05.006 [Crossref] [ Google Scholar]
- Olgun B, Oğuz G, Kaya M, Savlı S, Eskiçırak HE, Güney İ. The effects of magnesium sulphate on desflurane requirement, early recovery and postoperative analgesia in laparascopic cholecystectomy. Magnes Res 2012; 25(2):72-8. doi: 10.1684/mrh.2012.0315 [Crossref] [ Google Scholar]
- Ryu JH, Kang MH, Park KS, Do SH. Effects of magnesium sulphate on intraoperative anaesthetic requirements and postoperative analgesia in gynaecology patients receiving total intravenous anaesthesia. Br J Anaesth 2008; 100(3):397-403. doi: 10.1093/bja/aem407 [Crossref] [ Google Scholar]
- Asadollah S, Vahdat M, Yazdkhasti P, Nikravan N. The effect of magnesium sulphate on postoperative analgesia requirements in gynecological surgeries. Turk J Obstet Gynecol 2015; 12(1):34-7. doi: 10.4274/tjod.02439 [Crossref] [ Google Scholar]
- Wilder-Smith CH, Knöpfli R, Wilder-Smith OH. Perioperative magnesium infusion and postoperative pain. Acta Anaesthesiol Scand 1997; 41(8):1023-7. doi: 10.1111/j.1399-6576.1997.tb04830.x [Crossref] [ Google Scholar]
- Kiran S, Gupta R, Verma D. Evaluation of a single-dose of intravenous magnesium sulphate for prevention of postoperative pain after inguinal surgery. Indian J Anaesth 2011; 55(1):31-5. doi: 10.4103/0019-5049.76605 [Crossref] [ Google Scholar]
- Tsaousi G, Nikopoulou A, Pezikoglou I, Birba V, Grosomanidis V. Implementation of magnesium sulphate as an adjunct to multimodal analgesic approach for perioperative pain control in lumbar laminectomy surgery: a randomized placebo-controlled clinical trial. Clin Neurol Neurosurg 2020; 197:106091. doi: 10.1016/j.clineuro.2020.106091 [Crossref] [ Google Scholar]
- El Mourad MB, Arafa SK. Effect of intravenous versus intraperitoneal magnesium sulfate on hemodynamic parameters and postoperative analgesia during laparoscopic sleeve gastrectomy-a prospective randomized study. J Anaesthesiol Clin Pharmacol 2019; 35(2):242-7. doi: 10.4103/joacp.JOACP_208_18 [Crossref] [ Google Scholar]
- Samir EM, Badawy SS, Hassan AR. Intrathecal vs intravenous magnesium as an adjuvant to bupivacaine spinal anesthesia for total hip arthroplasty. Egypt J Anaesth 2013; 29(4):395-400. doi: 10.1016/j.egja.2013.06.004 [Crossref] [ Google Scholar]
- Kumar M, Dayal N, Rautela RS, Sethi AK. Effect of intravenous magnesium sulphate on postoperative pain following spinal anesthesia A randomized double blind controlled study. Middle East J Anaesthesiol 2013; 22(3):251-6. [ Google Scholar]
- Haryalchi K, Abedinzade M, Khanaki K, Mansour Ghanaie M, Mohammad Zadeh F. Whether preventive low dose magnesium sulphate infusion has an influence on postoperative pain perception and the level of serum beta-endorphin throughout the total abdominal hysterectomy. Rev Esp Anestesiol Reanim (Engl Ed) 2017; 64(7):384-90. doi: 10.1016/j.redare.2017.05.006 [Crossref] [ Google Scholar]
- Kayalha H, Yaghoubi S, Yazdi Z, Izadpanahi P. Effect of intervenous magnesium sulfate on decreasing opioid requirement after surgery of the lower limb fracture by spinal anesthesia. Int J Prev Med 2019; 10:57. doi: 10.4103/ijpvm.IJPVM_320_17 [Crossref] [ Google Scholar]
- Taheri A, Haryalchi K, Mansour Ghanaie M, Habibi Arejan N. Effect of low-dose (single-dose) magnesium sulfate on postoperative analgesia in hysterectomy patients receiving balanced general anesthesia. Anesthesiol Res Pract 2015; 2015:306145. doi: 10.1155/2015/306145 [Crossref] [ Google Scholar]
- Ghaffaripour S, Mahmoudi H, Eghbal H, Rahimi A. The effect of intravenous magnesium sulfate on post-operative analgesia during laminectomy. Cureus 2016; 8(6):e626. doi: 10.7759/cureus.626 [Crossref] [ Google Scholar]
- Bhatia A, Kashyap L, Pawar DK, Trikha A. Effect of intraoperative magnesium infusion on perioperative analgesia in open cholecystectomy. J Clin Anesth 2004; 16(4):262-5. doi: 10.1016/j.jclinane.2003.08.012 [Crossref] [ Google Scholar]
- Mentes O, Harlak A, Yigit T, Balkan A, Balkan M, Cosar A. Effect of intraoperative magnesium sulphate infusion on pain relief after laparoscopic cholecystectomy. Acta Anaesthesiol Scand 2008; 52(10):1353-9. doi: 10.1111/j.1399-6576.2008.01816.x [Crossref] [ Google Scholar]
- Koinig H, Wallner T, Marhofer P, Andel H, Hörauf K, Mayer N. Magnesium sulfate reduces intra- and postoperative analgesic requirements. Anesth Analg 1998; 87(1):206-10. doi: 10.1097/00000539-199807000-00042 [Crossref] [ Google Scholar]
- Mahajan C, Mishra RK, Jena BR, Kapoor I, Prabhakar H, Rath GP. Effect of magnesium and lignocaine on post-craniotomy pain: a comparative, randomized, double blind, placebo-controlled study. Saudi J Anaesth 2019; 13(4):299-305. doi: 10.4103/sja.SJA_837_18 [Crossref] [ Google Scholar]
- Bačak Kocman I, Krobot R, Premuzić J, Kocman I, Stare R, Katalinić L. The effect of preemptive intravenous low-dose magnesium sulfate on early postoperative pain after laparoscopic cholecystectomy. Acta Clin Croat 2013; 52(3):289-94. [ Google Scholar]
- Menshawi MA, Fahim HM. Dexmedetomidine versus magnesium sulfate as adjunct to general anesthesia in patients undergoing video-assisted thoracoscopy. Ain Shams J Anesthesiol 2022; 14(1):1-10. doi: 10.1186/s42077-021-00209-8 [Crossref] [ Google Scholar]
- Benevides ML, Fialho DC, Linck D, Oliveira AL, Ramalho DH, Benevides MM. Intravenous magnesium sulfate for postoperative analgesia after abdominal hysterectomy under spinal anesthesia: a randomized, double-blind trial. Braz J Anesthesiol 2021; 71(5):498-504. doi: 10.1016/j.bjane.2021.01.008 [Crossref] [ Google Scholar]
- Demiroglu M, Ün C, Ornek DH, Kıcı O, Yıldırım AE, Horasanlı E. The effect of systemic and regional use of magnesium sulfate on postoperative tramadol consumption in lumbar disc surgery. Biomed Res Int 2016; 2016:3216246. doi: 10.1155/2016/3216246 [Crossref] [ Google Scholar]
- Shin HJ, Kim EY, Na HS, Kim TK, Kim MH, Do SH. Magnesium sulphate attenuates acute postoperative pain and increased pain intensity after surgical injury in staged bilateral total knee arthroplasty: a randomized, double-blinded, placebo-controlled trial. Br J Anaesth 2016; 117(4):497-503. doi: 10.1093/bja/aew227 [Crossref] [ Google Scholar]
- Moon S, Lim S, Yun J, Lee W, Kim M, Cho K. Additional effect of magnesium sulfate and vitamin C in laparoscopic gynecologic surgery for postoperative pain management: a double-blind randomized controlled trial. Anesth Pain Med (Seoul) 2020; 15(1):88-95. doi: 10.17085/apm.2020.15.1.88 [Crossref] [ Google Scholar]
- Mavrommati PD, Gabopoulou ZT, Papadimos CN, Petsikopoulos MG, Vrettou VA, Konstantinidou MG. The perioperative infusion of low doses of magnesium sulfate reduces analgesic requirements in patients undergoing abdominal hernioplasty. Acute Pain 2004; 5(3-4):81-7. doi: 10.1016/j.acpain.2004.01.002 [Crossref] [ Google Scholar]
- Kizilcik N, Koner O. Magnesium sulfate reduced opioid consumption in obese patients undergoing sleeve gastrectomy: a prospective, randomized clinical trial. Obes Surg 2018; 28(9):2783-8. doi: 10.1007/s11695-018-3243-7 [Crossref] [ Google Scholar]
- Choi IG, Choi YS, Kim YH, Min JH, Chae YK, Lee YK. The effects of postoperative brachial plexus block using MgSO4 on the postoperative pain after upper extremity surgery. Korean J Pain 2011; 24(3):158-63. doi: 10.3344/kjp.2011.24.3.158 [Crossref] [ Google Scholar]
- Kaya S, Kararmaz A, Gedik R, Turhanoğlu S. Magnesium sulfate reduces postoperative morphine requirement after remifentanil-based anesthesia. Med Sci Monit 2009; 15(2):PI5-9. [ Google Scholar]
- Jarahzadeh MH, Taghizadeh Harati S, Babaeizadeh H, Yasaei E, Rahimi Bashar F. The effect of intravenous magnesium sulfate infusion on reduction of pain after abdominal hysterectomy under general anesthesia: a double-blind, randomized clinical trial. Electron Physician 2016; 8(7):2602-6. doi: 10.19082/2602 [Crossref] [ Google Scholar]
- Hwang JY, Na HS, Jeon YT, Ro YJ, Kim CS, Do SH. IV infusion of magnesium sulphate during spinal anaesthesia improves postoperative analgesia. Br J Anaesth 2010; 104(1):89-93. doi: 10.1093/bja/aep334 [Crossref] [ Google Scholar]
- Ebrahimy Dehkordy M, Tavanaei R, Younesi E, Khorasanizade S, Azizi Farsani H, Oraee-Yazdani S. Effects of perioperative magnesium sulfate infusion on intraoperative blood loss and postoperative analgesia in patients undergoing posterior lumbar spinal fusion surgery: a randomized controlled trial. Clin Neurol Neurosurg 2020; 196:105983. doi: 10.1016/j.clineuro.2020.105983 [Crossref] [ Google Scholar]
- Dabbagh A, Elyasi H, Razavi SS, Fathi M, Rajaei S. Intravenous magnesium sulfate for post-operative pain in patients undergoing lower limb orthopedic surgery. Acta Anaesthesiol Scand 2009; 53(8):1088-91. doi: 10.1111/j.1399-6576.2009.02025.x [Crossref] [ Google Scholar]
- Ayoglu H, Karadeniz Ü, Kunduracilar Z, Ayoglu FN, Erdemli Ö. The analgesic effect of magnesium sulfate and ketamine in patients undergoing laparoscopic cholecystectomy. The Pain Clinic 2005; 17(1):45-53. doi: 10.1163/1568569053421771 [Crossref] [ Google Scholar]
- Amiri H, Ghodrati N, Nikuyeh M, Shams-Vahdati S, Jalilzadeh-Binazar M. Comparison of granisetron and metoclopramide in the treatment of pain and emesis in migraine patients: a randomized controlled trial study. Turk J Emerg Med 2017; 17(2):61-4. doi: 10.1016/j.tjem.2016.12.004 [Crossref] [ Google Scholar]
- Shams Vahdati S, Sarkhosh Khiavi R, Rajaei Ghafouri R, Adimi I. Evaluation of prevalence of low back pain among residents of Tabriz University of Medical Sciences in relation with their position in work. Turk J Emerg Med 2014; 14(3):125-9. doi: 10.5505/1304.7361.2014.79106 [Crossref] [ Google Scholar]