J Res Clin Med. 12:37.
doi: 10.34172/jrcm.34496
Original Article
Spreading of dengue virus infection cases in Malda district of West Bengal, India during COVID-19 pandemic situation (2020 to 2022): A cross-sectional study
Puranjay Saha Conceptualization, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, 2 
Poulami Saha Investigation, 2
Subhayan Das Gupta Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing, 1, 2 
Dwiptirtha Chattopadhyay Data curation, Project administration, Resources, Software, 3 
Subham Das Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, * 
Author information:
1Virus Research & Diagnostics Laboratory (VRDL), Department of Microbiology, Malda Medical College & Hospital, Malda
2Department of Microbiology, Malda Medical College and Hospital, Malda
3District Public Health Laboratory (DPHL), Malda Medical College & Hospital, Malda
Abstract
Introduction:
One of the most widespread arboviral infections is caused by the dengue virus (Family: Flaviviridae) having a positive sense single-stranded RNA genome. It causes common symptoms like fever with headache, rashes, and other associated features like thrombocytopenia. This study aims to determine the prevalence of dengue virus infection and serotype dominance in the Malda district, West Bengal, India.
Methods:
During January 2020 to December 2022, the fever cases visited out-patient department or admitted to in-patient department at Malda Medical College were assessed for the presence of dengue NS1 antigen (fever<5 days) along with dengue IgM (fever≥5 days) in serum by ELISA. Serotype(s) of the dengue NS1-positive patients was determined using RT-qPCR.
Results:
The results revealed NS1 positivity to be 2.94% in 2020, 6.50% in 2021 and an increase up to 7.36% in 2022. Dengue IgM positivity was 0.35% in 2020, 5.59% in 2021 and reached 10.89% in 2022. During this period, the male population was infected more than the female. In 2020, the age-group<15 years was mostly infected (43%), while the age-group of 15-30 years accounted for the highest rate in 2021 (38%) and 2022 (34%). The serotyping data revealed that DENV-2 was most prevalent (60%), while some mixed infections (10%) with DENV-1 and DENV-3, were also found.
Conclusion:
Dengue infection is a major concern in this region and the rate of infection is continuously increasing yearly. DENV-2 serotype was found most predominant. Appropriate prevention along with proper awareness among the population, is utmost necessary.
Keywords: Arbovirus, Dengue, Enzyme-linked immunosorbent assay
Copyright and License Information
© 2024 The Authors.
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
The authors received no specific funding for this work.
Introduction
The causative agent for Dengue fever is Dengue virus which belongs to the Flaviviridae family, genus Flavivirus. Aedes aegypti and Aedes albopictus are considered as the primary & secondary vector for the transmission of dengue virus.
1
According to the data of NVBDCP (National Vector Borne Disease Control Programme), an initiative of National Health Mission (NHM) more than 7.5 lakh DENV infections were reported during the period of 2017 to 2022 (up to October) with nearly 1100 deaths in all over India.
2
According to World Health Organization (WHO), from 2015 to 2019, DENV infection in South East Asia (SEA) increased by 46% and the death increased by almost 2%. Five countries (India, Indonesia, Myanmar, Sri Lanka and Thailand) of SEA are among the 30 most highly endemic countries in the world.
3
Mutation in DENV (in DENV1 & DENV3) is already being reported from West Bengal in which there are changes in the amino acid sequences that leads to changes in protein structure also. This is a probable way for evolution of new variants.
4
The transmission of dengue virus may have been carried out through two cycles that is sylvatic cycle which shows transmission in wild animal like non-human primates or by monkey-aedes-monkey and the other is human cycle.
5
This arbovirus is a positive sense single stranded RNA virus which possesses three structural proteins namely, capsid (C), membrane (M) [which also have membrane Precursor called PrM protein] and envelope (E) proteins and seven nonstructural proteins namely NS1, NS2A, NS2B, NS3, NS4A, NS4B AND NS5.
6
Depending on the clinical symptoms dengue virus infection can be classified as simple dengue fever (DF), considered as mild disease but may have massive bleeding, dengue haemorrhagic fever (DHF), in which the first clinical phase is quite similar with DF but the major concern is the increase in vascular permeability that is also called plasma leakage. The third type is dengue shock syndrome (DSS), which is almost similar with DFF but with considerably higher amount of plasma leakage which leads to shock syndrome.
7
This roughly spherical virus, which is approximately 50 nm, has four independent antigen related serotypes i.e. DENV-1, DENV-2, DENV-3, and DENV-4. Based on several changes in genome, these serotypes can also be divided into subtypes or lineages which is called genotypes. The reported genotypes for DENV-1 is three (I, II, III), for DENV-2 is six (Asian/American, Asian I, Asian II, Cosmopolitan, American & Sylvatic), for DENV-3 is five (I, II, III, IV, V), and for DENV-4 is also five (I, IIA, IIB, III & sylvatic).
8,9
The recent addition in dengue Serotypes is DENV-5, which was announced in 2013; however, the suspected patient samples were collected in 2007 from Malaysia.
10
In 1789, Benjamin Rush first coined the name “break bone fever” because of the symptoms of myalgia and arthralgia which is now popular as Dengue fever. Ever since, this disease presented as epidemics in many regions like Asia, Africa & North America. In 1990s, this viral disease was considered as the second most wide-spread mosquito borne infection after Malaria.
11
In America, Africa, Asia, and Australia almost 390 million cases of DF was reported, among which 100 million had clinical features and near about 1000 cases developed fatal DHF/DSS.
12-14
During this year, in various regions of the Americas, between epidemiological week (EW) 1 and EW 52 of 2022, a total of 3,125,367 cases of arboviral disease were reported. Of those, 2 811 433 (90.0 %) were dengue cases.
15
For 2020, 26 EU/EEA countries reported 1957 cases of dengue, of which 1 820 (93%) were confirmed.
16
The first clinical evidence of dengue like illness in India was recorded in 1780 from Chennai (Madras at that time) and 1963-64 was the year when virologically proven epidemics of Dengue fever had taken place in Kolkata (Calcutta at that time) and Eastern coast of India.
17
In 2012, Tamil Nadu and West Bengal reported the highest number of dengue cases in India and also notable numbers of cases were seen in Maharashtra, Kerala, Karnataka and Delhi.
18
Malda (25.1786° N, 88.2461° E) is the one the district of West Bengal where dengue fever is one of the predominant infections in post monsoon season as reported data shows that since 2013 to 2016 the infection of dengue virus in this district has continuously risen with 2016 showing a total 1102 number of dengue virus infection cases in this area.
19
According to the 2017 report of Integrated Disease Surveillance Programme (IDSP), notable numbers of Dengue fever were reported from several parts of West Bengal like, South 24 Parganas (reported case: 834), North 24 Parganas (reported case: 794), Malda (reported case: 493) and Nadia (reported case: 124).
20
The purpose of this study is to identify a comprehensive analysis of spreading of dengue virus infection in Malda district during COVID-19 pandemic which may help identify the timing, age and sex preponderance in this region.
Materials and Methods
This is a retrospective case study, carried out at Virus Research & Diagnostics Laboratory (VRDL), at the Department of Microbiology, Malda Medical College, which includes three-year (January 2020- December 2022) dengue positive cases in Malda Medical College (patients admitted in in-patient department with dengue like illness and visited out-patient department). This study includes the statistical analysis which leads to the determination of the pattern of spread of dengue virus infection in Malda district as well as the identification of the serotypes prevalent in the region. The following steps were involved in carrying out this study.
Screening of dengue NS1 and IgM positive cases
Symptomatic patients suspected of dengue infection were selected from various departments (both outdoor and inpatient) of Malda Medical College & Hospital and were sent to the Department of Microbiology of the institution. Based on the duration of fever, screening was done by enzyme-linked immunosorbent assay (ELISA) from serum samples of patients. For cases with fever of less than five days duration only antigen (NS1) screening and for fever more than five days, antigen as well as dengue IgM antibody screening were done. After determination of cut off value for positive samples (as kits from different companies were used for both diagnosis, calculation varies for cutoff determination) the patients were declared as either positive or negative.
Serotyping of dengue NS-1 positive sample
In the end of 2022, Dengue serotyping by using of RT-qPCR was initiated and during this short period, a limited number (n = 30) of dengue samples were tested. The method has been described below:
Isolation of virus RNA
For isolation of dengue virus RNA from serum samples, Qiagen QIAamp® Viral RNA Mini kit (Catalog 52906) was used. This spin column based RNA isolation technique certifies high quality ready to use RNA with complete removal of contaminants and inhibitors. In this technique, 140 µL of serum from each sample was taken and using this protocol, RNA was entrapped into the spin column QIAamp which possesses a silica membrane. Then, by using the elution buffer provided in the kit, the pure RNA was eluted out in a fresh Eppendorf tube that was used in next step.21
Serotype determination by RT-qPCR
Determination of DENV serotype was carried out using Hi-PCR® Dengue Serotyping Probe PCR Kit (MBPCR137) of HiMedia Laboratories Pvt. Ltd. RT-qPCR was performed using a program of 50 °C for 15 minutes (reverse transcription), a step of inhibition of reverse transcriptase, an initial denaturation at 95 °C for 2 minutes; followed by the real-time diagnosis during 40 cycles of denaturation at 95 °C for 15 seconds and primer annealing with the extension at 60 °C for 1 minute in Bio-Rad CFX 96 Maestro. The polyprotein gene was the target of the probe PCR to detect dengue virus serotypes 1, 2, 3, and 4 by using FAM, JOE, Texas Red, Cy5 channels, respectively, with the internal control in Cy5.5. According to the protocol, 45 repeated cycles were carried out and interpreted as Ct value ≤ 40 was considered detected and either > 40 or N/A as Not detected.22
Data analysis
Date tabulation and all gathered data were maintained in Microsoft Excel 2016 licensed package and all graphical interpretation as well as all calculation were also carried out in MS Excel.
Results
Reported dengue cases
Since January 2020 to December 2022, detectable Dengue NS1 & IgM cases in Malda are tabulated in Table 1. 2279 symptomatic Dengue NS1 and 561 Dengue IgM samples were screened (out of which reported NS1 positive: 67 and IgM positive: 02) in 2020. 3075 symptomatic Dengue NS1and 1699 Dengue IgM samples were screened (reported NS1 positive: 200 and IgM positive: 95) in 2021 and in 2022, 7646 symptomatic Dengue NS1and 4059 Dengue IgM samples were screened (reported NS1 positive: 563 and IgM positive: 442).
Table 1.
Monthly dengue positive cases (2020-2022)
|
Month and year
|
Monthly dengue NS1 sample tested
|
Monthly NS 1 positive
|
Monthly dengue IgM sample tested
|
Monthly dengue IgM positive
|
| Jan-20 |
206 |
7 |
144 |
0 |
| Feb-20 |
261 |
4 |
178 |
0 |
| Mar-20 |
205 |
0 |
148 |
1 |
| Apr-20 |
57 |
1 |
0 |
0 |
| May-20 |
61 |
0 |
0 |
0 |
| Jun-20 |
206 |
3 |
0 |
0 |
| Jul-20 |
148 |
2 |
0 |
0 |
| Aug-20 |
150 |
6 |
0 |
0 |
| Sep-20 |
198 |
6 |
0 |
0 |
| Oct-20 |
359 |
6 |
1 |
0 |
| Nov-20 |
278 |
20 |
62 |
0 |
| Dec-20 |
150 |
12 |
31 |
1 |
| Jan-21 |
109 |
8 |
0 |
0 |
| Feb-21 |
73 |
0 |
144 |
0 |
| Mar-21 |
127 |
0 |
465 |
0 |
| Apr-21 |
120 |
0 |
66 |
0 |
| May-21 |
45 |
0 |
45 |
0 |
| Jun-21 |
78 |
2 |
78 |
0 |
| Jul-21 |
403 |
20 |
142 |
4 |
| Aug-21 |
539 |
23 |
42 |
2 |
| Sep-21 |
447 |
36 |
224 |
5 |
| Oct-21 |
482 |
82 |
160 |
30 |
| Nov-21 |
352 |
16 |
203 |
31 |
| Dec-21 |
300 |
13 |
130 |
23 |
| Jan-22 |
198 |
16 |
56 |
3 |
| Feb-22 |
185 |
9 |
63 |
9 |
| Mar-22 |
327 |
30 |
101 |
23 |
| Apr-22 |
345 |
8 |
96 |
10 |
| May-22 |
380 |
10 |
157 |
7 |
| Jun-22 |
562 |
48 |
344 |
17 |
| Jul-22 |
661 |
19 |
394 |
23 |
| Aug-22 |
678 |
31 |
290 |
15 |
| Sep-22 |
1010 |
56 |
390 |
45 |
| Oct-22 |
1331 |
173 |
742 |
107 |
| Nov-22 |
1391 |
142 |
927 |
149 |
| Dec-22 |
578 |
21 |
499 |
34 |
Month-wise dengue NS1 positivity
In 2020, irrespective of number, significant number of dengue NS1 positive cases were reported in all the months (except May) with November showing the highest positive cases (No. of positive: 20, positivity percentage: 7.19); however, positivity percentage was highest in December 2020 (No. of positive: 12, positivity percentage: 8.00). In January 2021, 8 dengue NS1 positive cases were detected and then up to May, 2021, no positive cases were found. However, from June, 2021, the infection started to spread with its highest peak in October, 2021 (No. of positive: 82, positivity percentage: 17.01) and then slowed down in November and December 2021. Another phenomenon was observed in the year 2022, where each month showed dengue virus infection cases and October was again the highest peak month of the year (No. of positive: 173, positivity percentage: 13.00). However, notable positive cases also observed in March (No. of positive: 30, positivity percentage: 09.17), June (No. of positive: 48, positivity percentage: 08.54), August (no of Positive: 31, Positivity percentage: 04.57), and also in September (No. of positive: 56, positivity percentage: 05.54) (Figure 1).
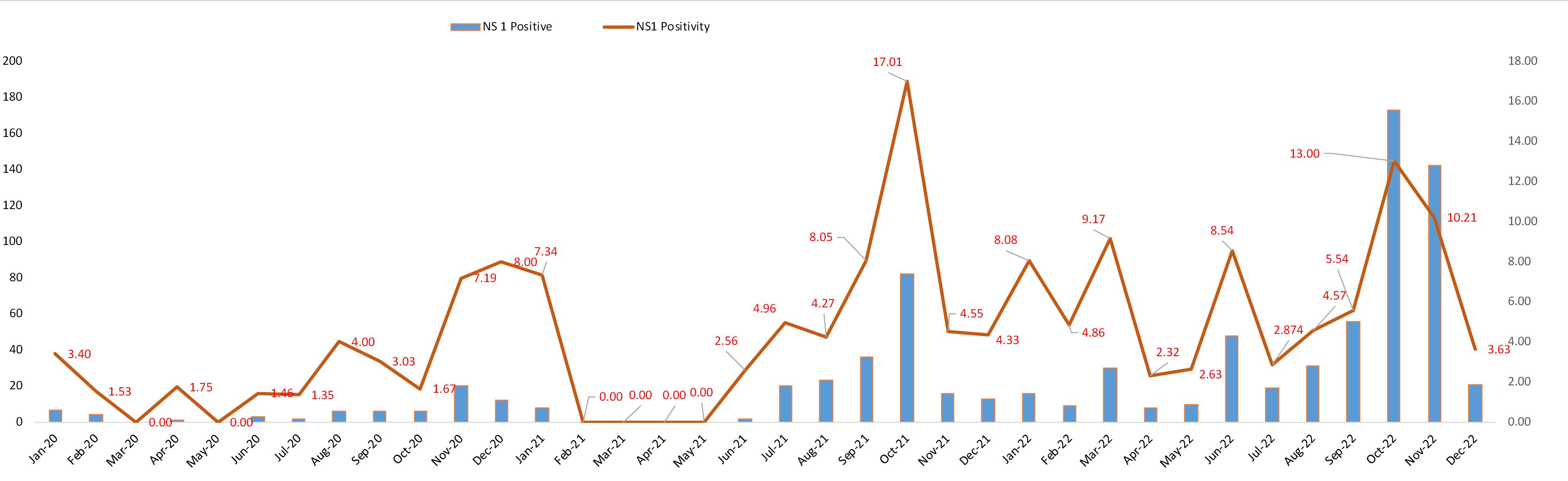
Figure 1.
Graphical representation of monthly dengue NS1 positive cases with positivity rate
.
Graphical representation of monthly dengue NS1 positive cases with positivity rate
Month wise dengue IgM positivity
During the first wave of COVID-19 pandemic (year 2020), comparatively fewer number of dengue IgM tests were carried out, and so the scenario was not clear in that year. Only two (one in March and another on December) positive cases was reported in that year. From July 2021 to December 2021, every months dengue IgM positive cases were reported and the highest positivity percentage was seen in October 2021 (No. of positive: 30, positivity percentage: 18.75) but the highest numbers of positive cases were reported in November 2021 (No. of positive: 31, positivity percentage: 15.27) along with notable positive cases in December 2021 (No. of positive: 23, positivity percentage: 17.69). Dengue IgM positives were reported throughout the year in 2022 along with the increased numbers of positive cases compared to previous two years. November included the highest positive cases (No. of positive: 149, positivity percentage: 16.07) along with notable positive cases also in October (No. of positive: 107, positivity percentage: 14.42) (Figure 2).
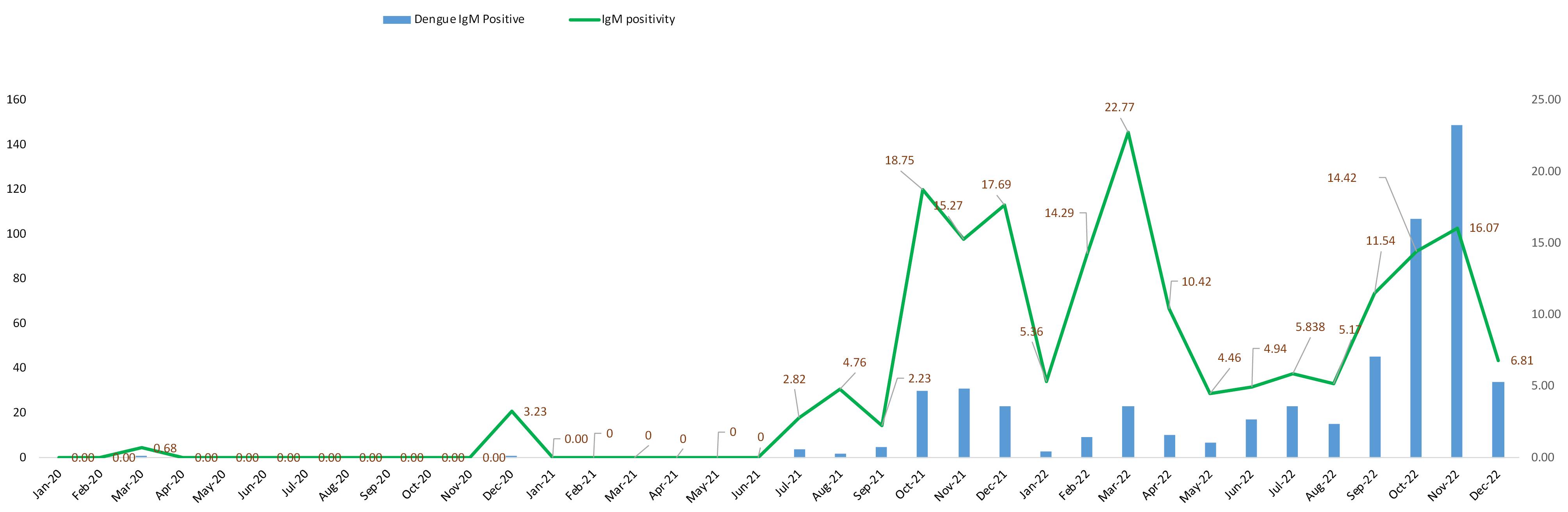
Figure 2.
Graphical representation of monthly dengue IgM positive cases with positivity rate
.
Graphical representation of monthly dengue IgM positive cases with positivity rate
Yearly positivity
From January 2020 to December 2022, the scenario was completely clear that DENV infection (NS1 & IgM detection) in this region had continuously risen which was a cause of severe public health concern. (Table 2 and Figure 3).
Table 2.
Yearly positivity percentages
|
Year
|
Dengue NS1 positivity (%)
|
Dengue IgM positivity (%)
|
| 2020 |
2.94 |
0.35 |
| 2021 |
6.50 |
5.59 |
| 2022 |
7.36 |
10.89 |
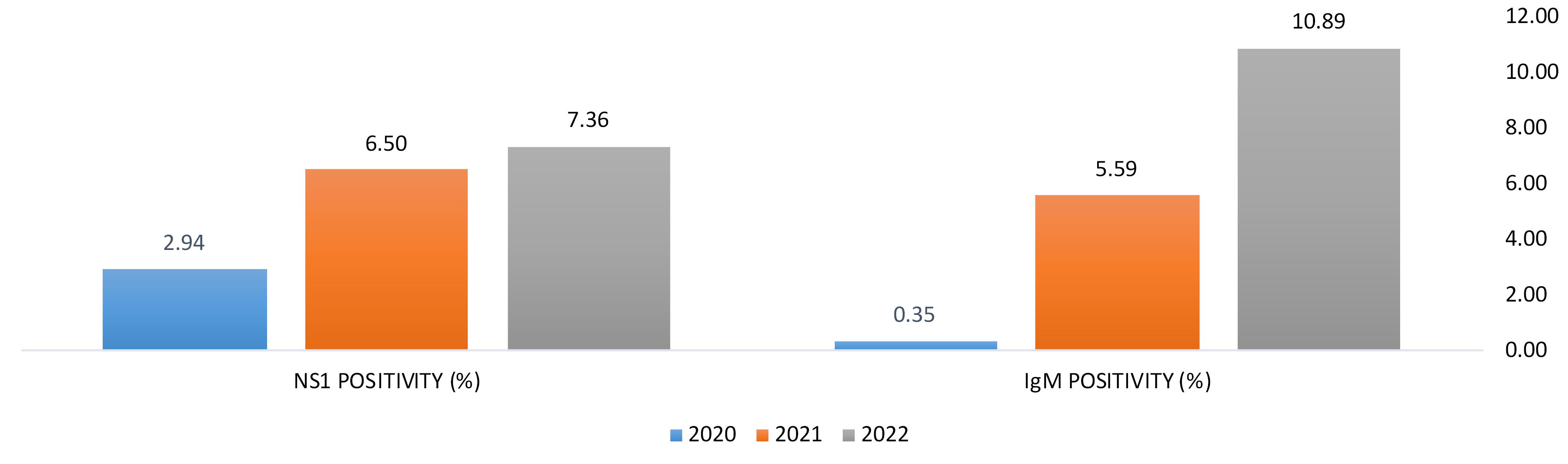
Figure 3.
Graphical representation of year wise rising cases of DENV infection
.
Graphical representation of year wise rising cases of DENV infection
Sex distribution of positive cases
The trends of emerging infection revealed that in every year, male population was infected more than female population. Also, the male infectivity is seen to be rising in every year as in 2020 only 1.89% of male patients were detected as dengue NS1 positive while that was increased to 3.71% in 2021 and then in 2022 it was raised to 5.80%. Similar behaviours were observed in the case of dengue IgM where the male positivity rate in 2020 was only 0.18 which was increased to 2.88% in 2021 and a huge 10.33% rise in 2022. In the case of female patients, although the overall positivity was lower than that of male patients but the incidence of infection among them had been also increased in every year (Table 3 and Figure 4).
Table 3.
Sex distribution of positive cases (both dengue NS1 & dengue IgM)
|
Year
|
Dengue NS1 male positivity (%)
|
Dengue NS1 female positivity (%)
|
Dengue IgM male positivity (%)
|
Dengue IgM female positivity (%)
|
| 2020 |
1.89 |
1.05 |
0.18 |
0.18 |
| 2021 |
3.71 |
2.80 |
2.88 |
2.71 |
| 2022 |
5.80 |
4.36 |
10.33 |
6.46 |
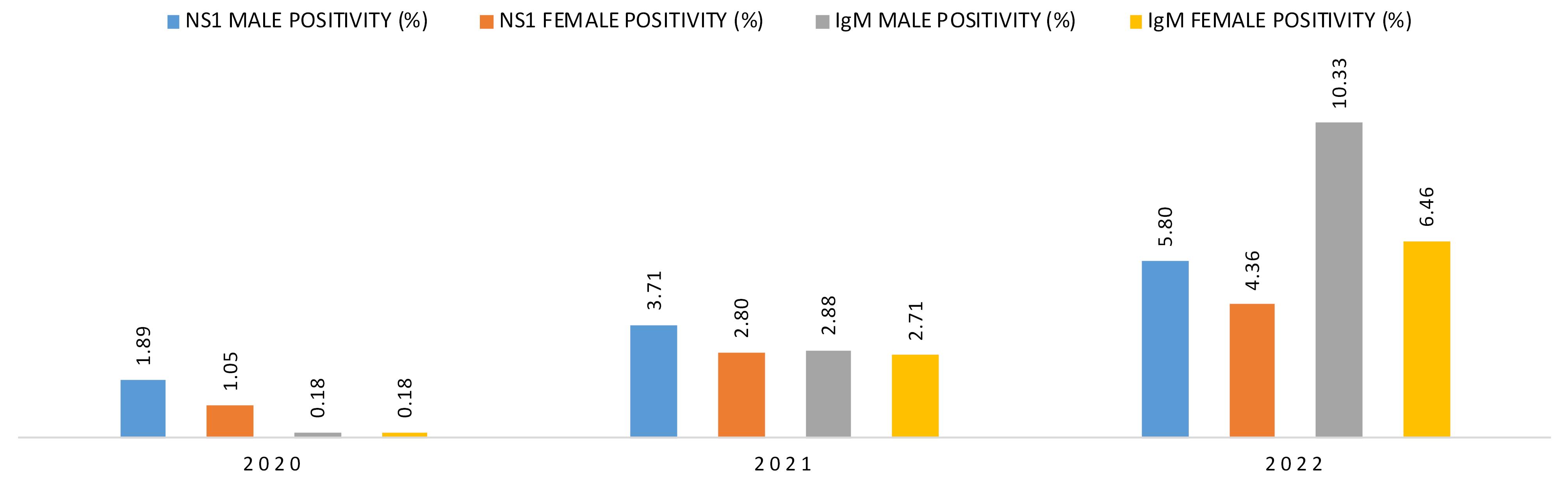
Figure 4.
Graphical representation of sex wise rising cases of DENV infection
.
Graphical representation of sex wise rising cases of DENV infection
Age distribution of positive cases
Distribution of cases according to age was another identifier for case dependent study by which we can identify which age group was infected more than the others.
Year 2020
According to this study, in 2020 most of the positive cases (n = 30), belonged to the age group < 15 years (43% of cases) followed by the age group belongs to 15-30 years (n = 20, 29% of cases) (Table 4 and Figure 5).
Table 4.
Positive case distribution in year 2020
| |
Age<15 years
|
Age 15-30 years
|
Age 31-45 years
|
Age 46-60 years
|
Age>60 years
|
| Total cases (in year 2020) |
30 |
20 |
10 |
8 |
1 |
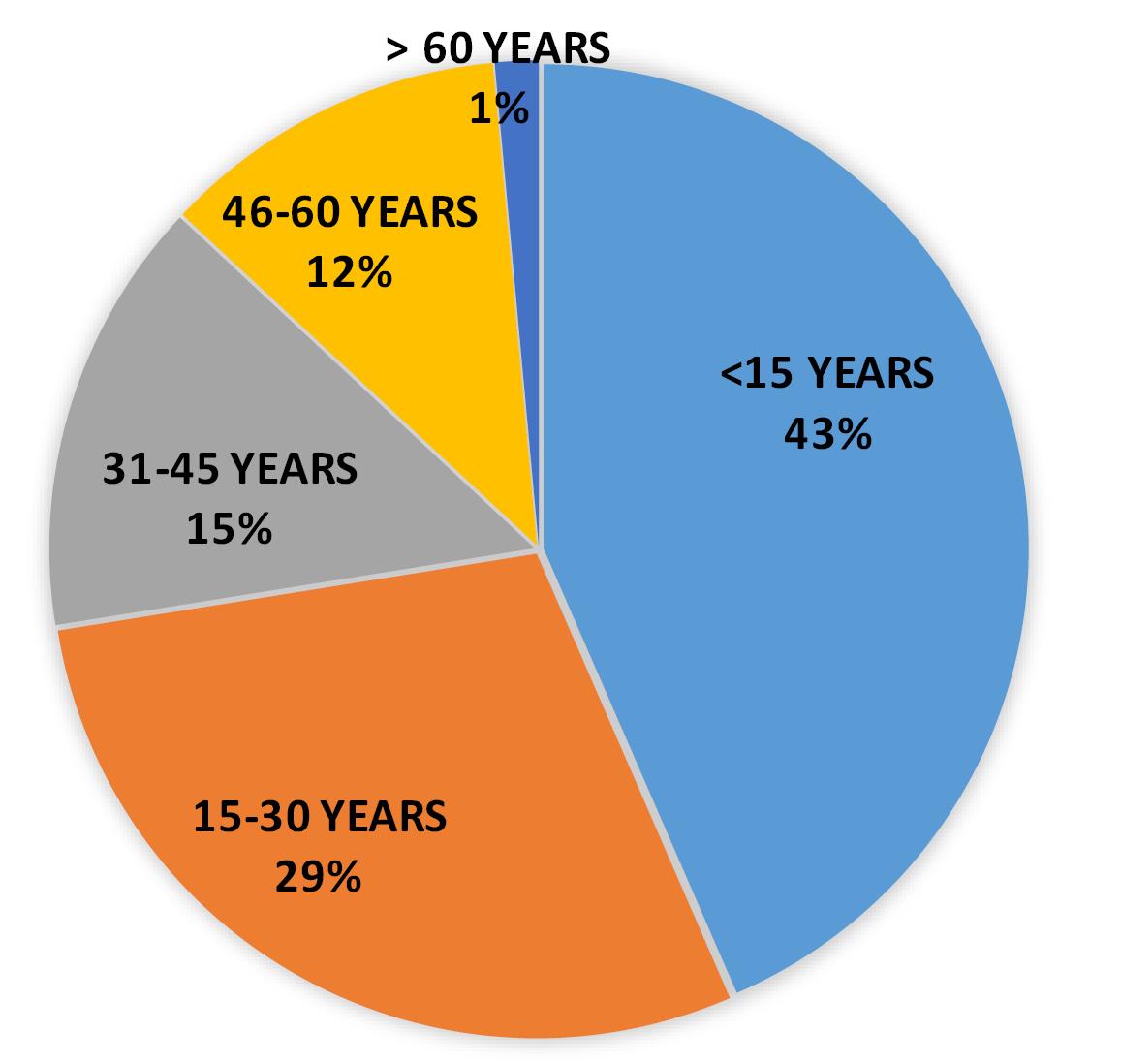
Figure 5.
Pie distribution of positive cases in 2020
.
Pie distribution of positive cases in 2020
Year 2021
The scenario was quite different in year 2021, where 38% of positive cases belonged to the age group 15-30 years (n = 110), however 30% of cases were patients whose age was less than 15 years (n = 86). However, notable positive cases were also reported from other age group like 31-45 (15% cases) and 46-60 years (14% cases) (Table 5 and Figure 6).
Table 5.
Positive case distribution in year 2021
| |
Age<15 years
|
Age 15-30 years
|
Age 31-45 years
|
Age 46-60 years
|
Age>60 years
|
| Total cases (in year 2021) |
86 |
110 |
45 |
40 |
10 |
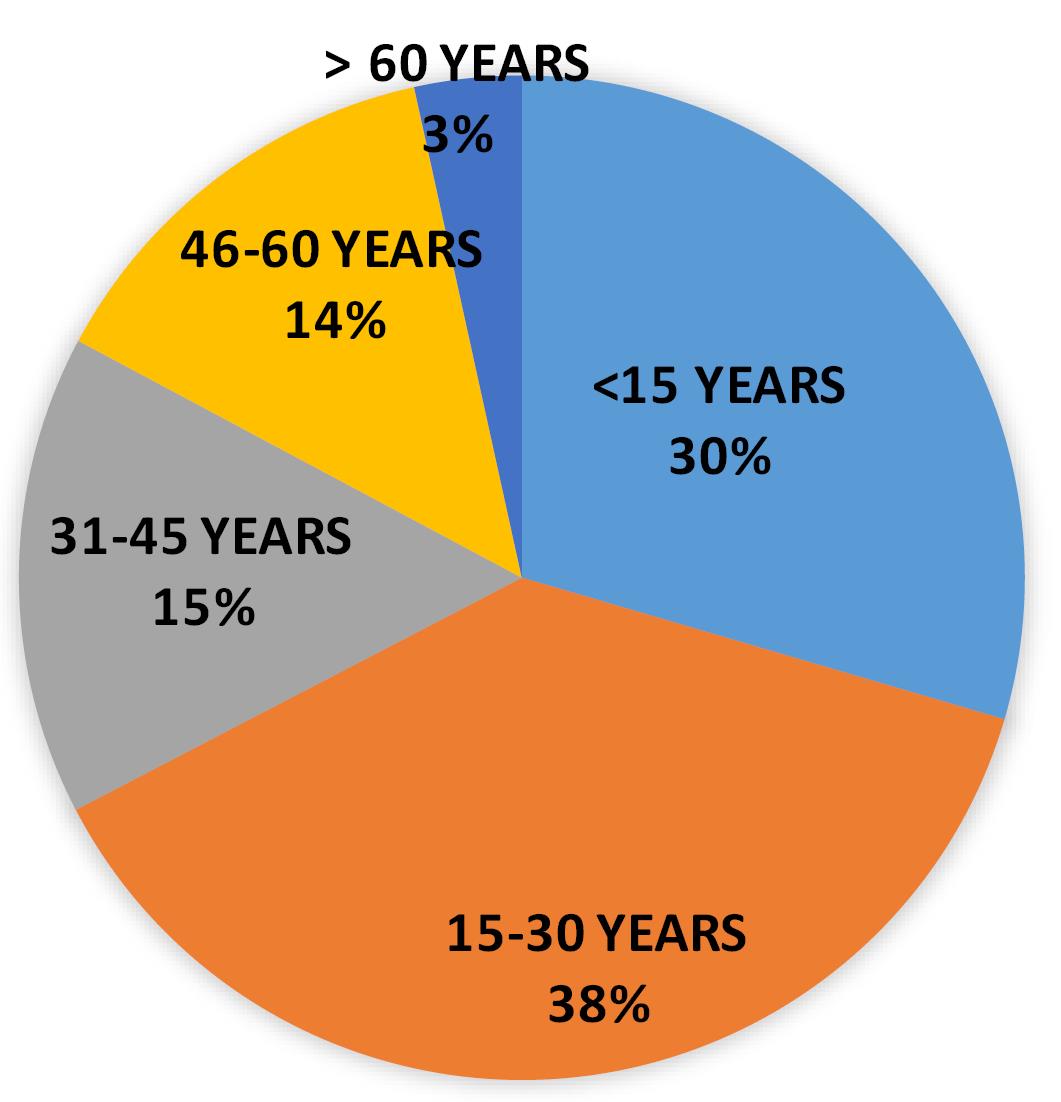
Figure 6.
Pie distribution of positive cases in 2021
.
Pie distribution of positive cases in 2021
Year 2022
The scenario of cases in 2022 was quite similar to 2021 where age group of 15-30 years was highly infected i.e. 34 % of cases (No. of cases: 311) followed by the cases belonging to the age group less than 15 years (No. of cases: 277) with 30% of the reported cases. But the important finding in 2022 was that DENV infection had affected almost all of the age groups with notable number of positive cases (Table 6 and Figure 7).
Table 6.
Positive case distribution in year 2022
| |
Age<15 years
|
Age 15-30 years
|
Age 31-45 years
|
Age 46-60 years
|
Age>60 years
|
| Total cases (in year 2022) |
277 |
311 |
184 |
92 |
53 |
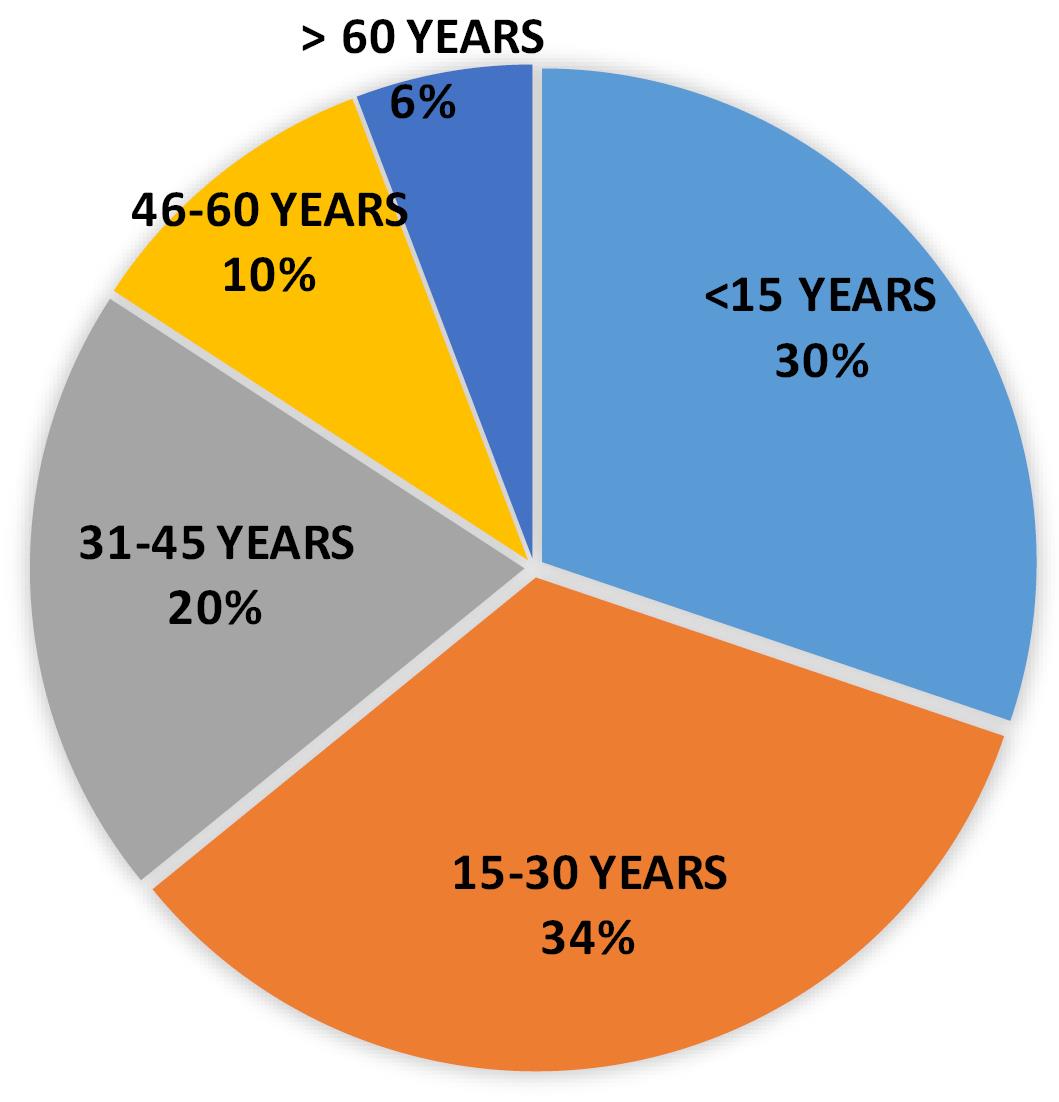
Figure 7.
Pie distribution of positive cases in 2022
.
Pie distribution of positive cases in 2022
Dengue serotype prevalence
The most recent study that was carried out in our laboratory was, to determine the dengue serotype prevalence in this region. By using RT-qPCR technique, result revealed that, most of DENV infection was due to the DENV2 serotype with 60% of the reported cases belonging to this group. 20% of the cases were due to the infection of DENV3 serotype and most interestingly a combined infection of DENV1 & DENV3 was also found with the 10% of the cases. We did not get any DENV1 infection within study sample. Interestingly 10% of the samples were DENV RNA negative. (Table 7 and Figure 8) We were not sure about the reason of DENV (which were Dengue NS1 ELISA positive) RNA negative cases. However, this study had its own limitation regarding to the size of the sample so we can say this could be an initial trend of this study. Whenever, we will able to do more and more serotyping through RT-qPCR, the actual scenario will become crystal clear.
Table 7.
DENV serotype prevalence in 2022
| |
DENV1 prevalence (%)
|
DENV2 prevalence (%)
|
DENV3 prevalence (%)
|
DENV4 prevalence (%)
|
DENV1 & DENV3 prevalence (%)
|
RNA negative prevalence (%)
|
| Percentage positivity |
0 |
60 |
20 |
0 |
10 |
10 |
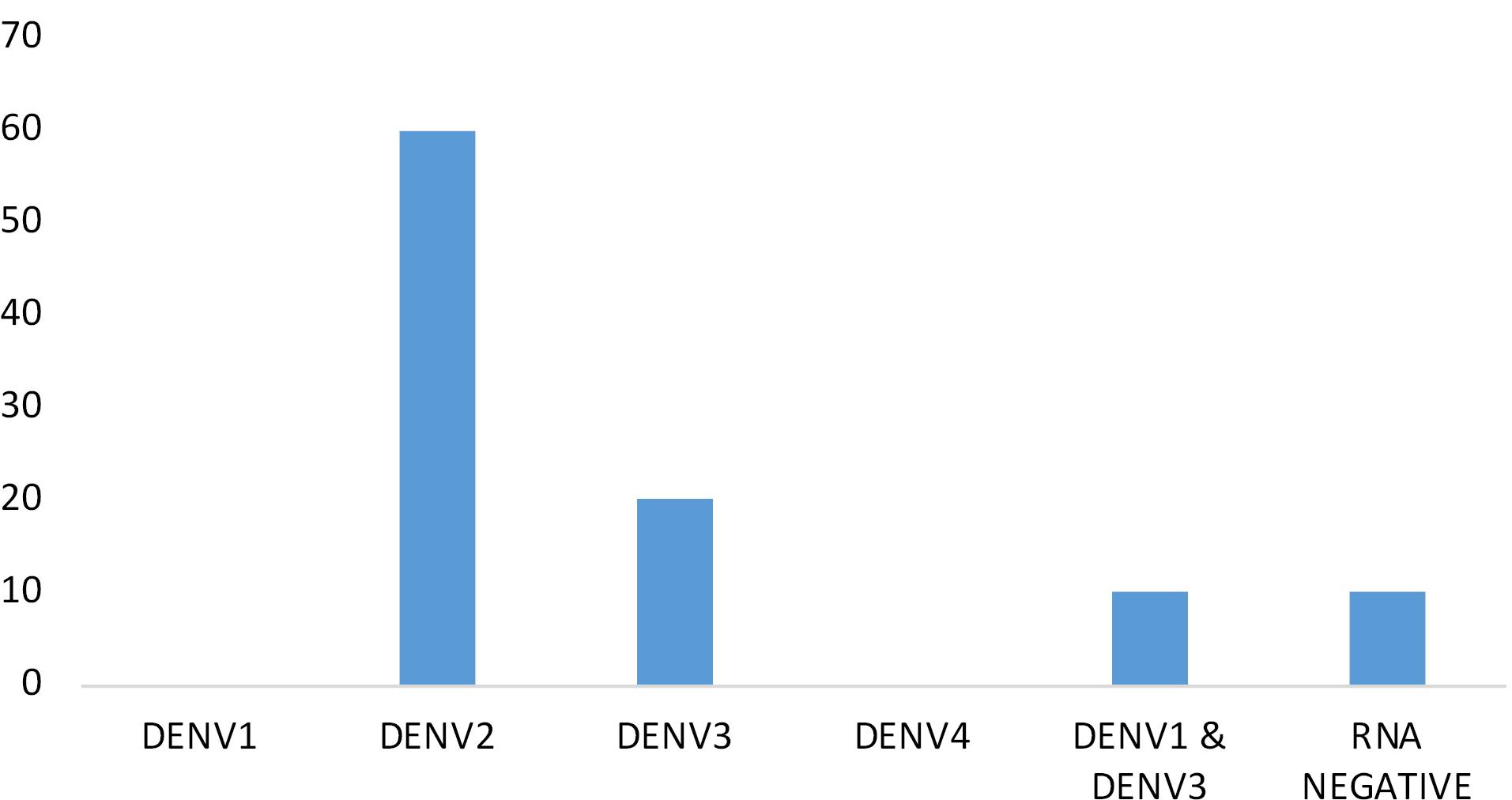
Figure 8.
Graphical representation of DENV serotype prevalence
.
Graphical representation of DENV serotype prevalence
Discussion
Dengue the most infective arbovirus belonging to the Flaviviridae family. It is one of the major public health concerns in India. Since 1963-64, this viral infection is continuously rising in West Bengal23 along with it being a major public health concern in many places like Tamil Nadu, Maharashtra, Kerala, Gujrat, Puducherry and Haryana.18
In the three years of this study (2020-2022), it was clearly observed that during the COVID-19 pandemic, the spread of dengue virus infection was also increased in this region. Data reveals that symptomatic patients as well as DENV infection was continuously rising (Dengue NS1 positivity of 2.94% increased to 7.36% in 2022 and Dengue IgM positivity increased from 0.35% in 2020 to 10.89% in 2022) in this region. The patients with age-group 15-30 years corresponds 29% of Dengue NS1 infection in 2020, 38% in 2021 and 34% in 2022), however, the age -group < 15 years also showed notable number of cases (43% in 2020, 30% in 2021, 30% 2022) with a complete male population dominancy over females. This result corroborates the findings of Bandyopadhyay et al,18 and Dinkar & Singh24 as they had also reported that the male population up to age limit of 30 years was the main infected population. According to Chakravarti and Kumaria,25 and Gupta et al,26 male population was the most infected population along with the age groups belonging to 21-30 years. These studies comparing to ours, clearly reveals that the young male population is the main victim of dengue virus infection cases. However, a study by Murhekar et al,27 showed quite different pattern like age group belonged 9-17 years showed highest positive cases along with highest female patients’ dominancy over male patients.
According to Debnath et al28 and Bandyopadhyay et al18 all of the dengue serotypes like DENV1, DENV2, DENV3 and DENV 4 have been presented in West Bengal and among them DENV 1 infection is predominant. Mixed serotype infection of dengue virus from the northern part of West Bengal was already been reported by Roy et al.3 In the present study, at Malda district (at the junction of the northern and southern part of West Bengal) DENV2 was found to be the predominant one, and another alarming finding in this study was that patients are being infected with DENV 3 serotype and also with DENV 1& DENV3 coinfection. However, a recent study by Batool et al29 showed that during and after COVID-19 pandemics, DENV2 is the predominant serotypes which was completely correlates with our study.
Study Highlights
What is current knowledge?
-
Malda is one of the district of West Bengal where dengue fever is one of the predominant infections in post monsoon season as reported data shows that since 2013 to 2016 the infection of dengue virus in this district has continuously risen with 2016 showing a total 1102 number of dengue virus infection cases in this area. According to the 2017 report of Integrated Disease Surveillance Programme (IDSP), notable numbers of Dengue fever were reported from several parts of West Bengal that includes Malda (reported case: 493) also but there was no clear indication of predominant serotype of Dengue virus.
What is new here?
Conclusion
From this study, it was clearly observed that, DENV infection is a rising concern in this region and year by year the percentage of infection is also continuously increasing. Proper prevention as well as proper awareness among the population is utmost necessary things as the infection targeting all age groups (irrespective of numbers). If this kind of coinfection starts hiking in number, this will create another tough condition to handle in the public health scenario. Last but not the least, timely identification and determination of the serotype constantly spreading is the only way to control this deadly infection.
Acknowledgements
We are highly acknowledging all faculty & staff of Department of Microbiology, Malda Medical College & Hospital as well as all other staff of Malda Medical college. Without their help this study would not be possible. Also, Indian Council of Medical Research (ICMR) and Department of Health Research (DHR), Govt. of India for funding us to established Virus Research & Diagnostics Laboratory (VRDL) and Department of health & family Welfare, Govt. of West Bengal for providing us place in Malda Medical College for establishment of this BS level 2 Laboratory.
Competing Interests
None.
Ethical Approval
Not applicable.
References
- Carrington LB, Simmons CP. Human to mosquito transmission of dengue viruses. Front Immunol 2014; 5:290. doi: 10.3389/fimmu.2014.00290 [Crossref] [ Google Scholar]
- National Center for Vector Borne Diseases Control (NCVBDC), Ministry of Health & Family Welfare, Government of India. Dengue Situation in India. NCVBDC; 2024.
- World Health Organization (WHO). Dengue Fact Sheet. Available from: https://www.who.int/southeastasia/health-topics/dengue-and-severe-dengue.
- Roy SK, Goswami BK, Bhattacharjee S. Genetic characterization of dengue virus from patients presenting multi-serotypic infections in the Northern West Bengal, India. Virus Genes 2023; 59(1):45-54. doi: 10.1007/s11262-022-01950-4 [Crossref] [ Google Scholar]
- Vasilakis N, Cardosa J, Hanley KA, Holmes EC, Weaver SC. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol 2011; 9(7):532-41. doi: 10.1038/nrmicro2595 [Crossref] [ Google Scholar]
- Perera R, Kuhn RJ. Structural proteomics of dengue virus. Curr Opin Microbiol 2008; 11(4):369-77. doi: 10.1016/j.mib.2008.06.004 [Crossref] [ Google Scholar]
- Kalayanarooj S. Clinical manifestations and management of dengue/DHF/DSS. Trop Med Health 2011; 39(4 Suppl):83-7. doi: 10.2149/tmh.2011-S10 [Crossref] [ Google Scholar]
- Chen R, Vasilakis N. Dengue--quo tu et quo vadis?. Viruses 2011; 3(9):1562-608. doi: 10.3390/v3091562 [Crossref] [ Google Scholar]
- Zerfu B, Kassa T, Legesse M. Epidemiology, biology, pathogenesis, clinical manifestations, and diagnosis of dengue virus infection, and its trend in Ethiopia: a comprehensive literature review. Trop Med Health 2023; 51(1):11. doi: 10.1186/s41182-023-00504-0 [Crossref] [ Google Scholar]
- Mustafa MS, Rasotgi V, Jain S, Gupta V. Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control. Med J Armed Forces India 2015; 71(1):67-70. doi: 10.1016/j.mjafi.2014.09.011 [Crossref] [ Google Scholar]
- Dengue Virus Net. Dengue Epidemiology [Internet]. Dengue Virus Net. 2022. Available from: http://www.denguevirusnet.com/epidemiology.html. Accessed September 5, 2022.
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL. The global distribution and burden of dengue. Nature 2013; 496(7446):504-7. doi: 10.1038/nature12060 [Crossref] [ Google Scholar]
- Ross TM. Dengue virus. Clin Lab Med 2010; 30(1):149-60. doi: 10.1016/j.cll.2009.10.007 [Crossref] [ Google Scholar]
- Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 2016; 16(6):712-23. doi: 10.1016/s1473-3099(16)00026-8 [Crossref] [ Google Scholar]
- Pan American Health Organization (PAHO) website. Available from: https://www.paho.org/plisa.
- European Centre for Disease Prevention and Control. Dengue - Annual Epidemiological Report for 2020. Available from: https://www.ecdc.europa.eu/en/publications-data/dengue-annual-epidemiological-report-2020.
- Gupta N, Srivastava S, Jain A, Chaturvedi UC. Dengue in India. Indian J Med Res 2012; 136(3):373-90. [ Google Scholar]
- Bandyopadhyay B, Bhattacharyya I, Adhikary S, Konar J, Dawar N, Sarkar J. A comprehensive study on the 2012 dengue fever outbreak in Kolkata, India. Int Sch Res Notices 2013; 2013(1):207580. doi: 10.5402/2013/207580 [Crossref] [ Google Scholar]
- Mandal AK, Mondal T, Saha P, Saha P, Roy A, Kundu PK. An insight investigation of dengue in a tertiary care teaching hospital, West Bengal. J Pure Appl Microbiol 2017; 11(3):1617-22. doi: 10.22207/jpam.11.3.49 [Crossref] [ Google Scholar]
- District wise disease alerts/outbreaks reported in the 38th Week, 2017. Available from: https://www.idsp.mohfw.gov.in/WriteReadData/l892s/382017.pdf.
- QIAamp Viral RNA Kits for RNA Extraction. Available from: https://www.qiagen.com/us/products/diagnostics-and-clinical-research/sample-processing/qiaamp-viral-rna-kits.
- Ranjan M, Shveta Shveta, Kawatra S, Singh S, Agrawal A, Sharma A. Dengue detection and serotyping using multiplex real time polymerase chain reaction: study from a tertiary care centre in Eastern India. Eur J Mol Clin Med 2022; 9(2):1475-81. [ Google Scholar]
- Aikat BK, Konar NR, Banerjee G. Haemorrhagic fever in Calcutta area. Indian J Med Res 1964; 52:660-75. [ Google Scholar]
- Dinkar A, Singh J. Dengue infection in North India: an experience of a tertiary care center from 2012 to 2017. Tzu Chi Med J 2020; 32(1):36-40. doi: 10.4103/tcmj.tcmj_161_18 [Crossref] [ Google Scholar]
- Chakravarti A, Kumaria R. Eco-epidemiological analysis of dengue infection during an outbreak of dengue fever, India. Virol J 2005; 2:32. doi: 10.1186/1743-422x-2-32 [Crossref] [ Google Scholar]
- Gupta E, Dar L, Kapoor G, Broor S. The changing epidemiology of dengue in Delhi, India. Virol J 2006; 3:92. doi: 10.1186/1743-422x-3-92 [Crossref] [ Google Scholar]
- Murhekar MV, Kamaraj P, Kumar MS, Khan SA, Allam RR, Barde P. Burden of dengue infection in India, 2017: a cross-sectional population based serosurvey. Lancet Glob Health 2019; 7(8):e1065-73. doi: 10.1016/s2214-109x(19)30250-5 [Crossref] [ Google Scholar]
- Debnath F, Provash CS, Chakraborty A, Dutta S. Dengue fever outbreak by more than one serotype in a municipal area of Kolkata, Eastern India. J Vector Borne Dis 2019; 56(4):380-2. doi: 10.4103/0972-9062.302043 [Crossref] [ Google Scholar]
- Batool A, Kanwal N, Akram M, Rauf S, Navid MT, Masood F, et al. Molecular characterization and expression analysis of NS3 and NS4 genes of dengue virus serotype-2 from Pakistani isolates. Res Sq [Preprint]. December 23, 2023 Available from: https://www.researchsquare.com/article/rs-3732242/v1.