J Res Clin Med. 12:21.
doi: 10.34172/jrcm.34492
Original Article
Dermatologic manifestations of patients with severe and non-severe COVID-19 infection: A cross-sectional study
Mehrad Amirnia Conceptualization, Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing, 1 
Mehdi Haghdoost Investigation, Validation, 2 
Khazar Raeisnia Data curation, Investigation, Writing – original draft, 1 
Asal Raeisnia Methodology, Writing – original draft, 1 
Seyyed Sina Hejazian Formal analysis, Software, Writing – original draft, Writing – review & editing, 3 
Mehdi Amirnia Conceptualization, Investigation, Project administration, Resources, Supervision, Validation, 1, * 
Author information:
1Department of Dermatology, Sina Hospital, Tabriz, Iran
2Department of Infectious Disease, Imam Reza Hospital, Tabriz, Iran
3Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Since the emergence of COVID-19 in late 2019, multiple concerns have been raised regarding the unknown nature of the disease, including dermatologic complications. In this study, we discussed dermatologic manifestations of patients with COVID-19 infection who visited our clinic.
Methods:
Eighty- six patients were involved based on our inclusion and exclusion criteria (50 female and 36 male). All patients with definite diagnosis of COVID-19 who were referred to our dermatologic clinic were enrolled in this cross-sectional study. Only lesions which occurred 1 week prior to or 3 months after first COVID-19 symptoms were included. The photographs of all lesions were independently reviewed by four experienced dermatologists and an infectious disease specialist to reach the objectives stated above. Qualitative data presented as frequency (%) and compared using chi-squared or Fisher’s exact test, and quantitative data presented as mean±SD or median (IQR) based on normality, with P value≤0.05 considered statistically significant.
Results:
Eighty- six patients were enrolled in the study (mean±SD age=36.95±17.78). Eighteen specific types of dermatologic complications were seen. The most common lesions included maculopapular rash and scalp hair loss. The only lesions with a statistically significant difference regarding COVID-19 symptom onset time were maculopapular rash, telogen effluvium, and papular urticaria. The only lesions with statistically significant difference regarding COVID-19 severity were maculopapular rash, telogen effluvium, and urticaria.
Conclusion:
Our study revealed that a wide range of skin lesions may occur in patients with COVID-19. We found a statistically significant relation between disease severity and certain dermatologic manifestations.
Keywords: COVID-19, Communicable diseases, Skin diseases, Viral
Copyright and License Information
© 2024 The Authors.
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
The authors declare that this research was not funded and received no financial support from any sources.
Introduction
Late in 2019, an unknown disease with flu-like symptoms broke out in Wuhan-China. Later in March, the World Health Organization named this illness as coronavirus disease 2019 (COVID-19), recognizing it as a pandemic.1 The density of functional angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) receptors on the surface of organ cells determines the infection risk of any organ by COVID-19.2 Some studies have found that an increased level of ACE-2 and the use of immunosuppressants may elevate the risk of COVID-19 infection in patients with systemic lupus erythematosus.3 While ACE-2 is abundantly found on endothelial cells,4 suggesting that the dermatologic manifestations after COVID-19 may be related to endothelial cell dysfunction, microthrombosis, and skin tissue hypoxia,5 this receptor is infrequently expressed on the basal layer of the skin and hair follicles. Thus, the hypothesis of direct infection of skin cells has been questioned.5 In contrast, endothelial infection and inflammatory cytokine-release can cause vasodilation and rise of blood transfusion to the skin, triggering tissue edema and urticarial lesions. There are several factors that predict the severity of COVID-19 infection in affected patients, including inflammatory indices (e.g., dNLR).6 Regarding the effect of inflammatory factors on dermatologic manifestations, one of our aims was to examine the hypothesis of whether dermatologic findings could be indicators of COVID-19 severity. Based on previous studies, the prevalence of skin lesions after COVID-19 infection is approximately 6% and they generally appear within four weeks of COVID-19 infection. The most common dermatological manifestations of COVID-19 were maculopapular rashes, erythema multiform-like and pityriasis rosea.7 Less frequent lesions include erythema pernio like, vesiculobullous lesions, livedo reticularis, purpura, and acral cyanosis.8 Chilblain-like and vascular lesions (including petechiae and purpura, livedo reticularis and necrosis) with lowest and highest mortality rate, respectively, represent the two ends of the COVID-19-related skin lesion spectrum.7 The prevalence of skin lesions after COVID-19 infection varies based on racial differences, age, lifestyle, diet, and geographical parameters.9 Here, we reported the dermatologic manifestations of patients with a definite diagnosis of COVID-19 infection who were referred to our dermatology clinics. We only considered findings that occurred within one week prior to three months after the first COVID-19 symptom. Our objectives included evaluation of dermatologic findings of patients with different COVID-19 severities, the average time of occurrence for each lesion, and the average age of patients with each lesion. To the best of our knowledge, no similar study has been conducted in our region.
Methods
Methodology
This cross-sectional study was performed on patients with a definite diagnosis of COVID-19 infection who visited our specialized dermatology and infectious disease clinics located at Sina and Imam Reza hospital within a one-year period from April 2021 to April 2022 in Tabriz, Iran. Although a non-probability convenient sampling method was used due to the low prevalence of skin complications after COVID-19, we acknowledge the potential for selection bias and limitations in the generalizability of the findings. Only lesions which occurred 1 week prior to or 3 months after first COVID-19 symptoms were included. Data was collected using a proper questionnaire which included demographic features of patients (including age, job, address, comorbidities and recently used medications), method of COVID-19 diagnosis (PCR, chest CT scan), type, location, morphology and color of the lesion, time of occurrence (considering the time of first COVID-19 symptom as the zero-time origin), and patient’s chief dermatologic complaint. To ensure the accuracy of the dermatologic assessments, all skin lesions were evaluated using a dermatoscope. Additionally, all lesions were photographed with the patient’s permission. The photographs were independently reviewed by four experienced dermatologists, while rest of the clinical information was hidden from them. Finally, a consensus was reached on the diagnosis of the lesions.
The exclusion criteria consisted of chronic skin diseases (including psoriasis, cutaneous lichen planus, and pemphigus vulgaris), and suspected drug-related dermatologic complications within one week after drug consumption. Skin complications which were considered as hydroxychloroquine side effects included hyperpigmentation, acute generalized exanthematous pustulosis, and Stevens-Johnson syndrome.10 Severe skin reaction with fever, angioedema, red or purple generalized rashes, and bullae were considered as azithromycin-related skin lesion.
Skin lesions that occurred within the first 14 days of the inclusion period were considered early complications, while others were considered late complications. Patients with positive history of hospitalization due to COVID-19 infection and/or SPO2 < 90% at the first visit (based on the pulse-oximetry at the time of visit) were categorized as severe COVID-19 disease.
Statistical analysis
All data were analyzed by statistics expert using IBM SPSS statistics version 26. The qualitative data was shown as frequency (%) and their comparison between groups were performed using Chi-squared or Fisher’s exact test. Mean ± standard deviation (SD) or median (IQR) were used to show quantitative data based on the normality of the distribution. P value ≤ 0.05 was considered as statistical significance level.
Ethical considerations
In this study, we examined the dermatologic manifestations of COVID-19 in human subjects, following strict ethical guidelines and obtaining informed consent from each participant. The study protocol was approved by the institutional review board. All participants were carefully informed about the study’s objectives, possible risks, benefits, and their right to withdraw their consent at any point without any consequences. Only subjects who willingly provided written informed consent were enrolled in the research. Additionally, we ensured that all data obtained from the participants remained confidential throughout the research process.
Results
Demographic features and general findings
In this study, a total of 100 patients were initially included. However, based on the pre-defined exclusion criteria, 14 cases were subsequently excluded, leaving a final sample size of 86 patients. Demographic features of these patients are shown in Table 1. The mean ± SD age of patients was 36.95 ± 17.78 years old. The majority of patients were female (48, 59.3%). The most frequent comorbidities among patients were listed as hypertension (12 cases,14%), DM (10 cases, 11.6%), and ischemic heart disease (2 cases, 2.3%). 70 patients (81.4%) had positive PCR test for COVID-19 and 50 patients (58.1%) were hospitalized due to severe COVID-19 infection. Overall, 44 patients (51.8%) had severe COVID-19 infection (5 positive for SPO2% < 90%, 10 positive for hospital admission, and 29 positive for both criteria).
Table 1.
Demographic features and general findings regarding the patients
|
Characteristic (patients’ number=86)
|
Frequency
|
| Gender |
Male |
36 (41.9%) |
| Female |
50 (58.1%) |
| Age |
Mean ± SD |
36.95 ± 17.78 |
| Median (Interquartile range) |
39.5 (26-50) |
| Minimum-Maximum |
1-77 |
| Medical History |
Hypertension |
12 (14.0%) |
| Diabetes |
10 (11.6%) |
| IHD |
2 (2.3%) |
| Hyperlipidemia |
1 (1.2%) |
| Seizure |
1 (1.2%) |
| Thyroid dysfunction |
1 (1.2%) |
| Chronic heart disease |
1 (1.2%) |
| Minor thalassemia |
1 (1.2%) |
| IBD |
1 (1.2%) |
| Residency |
County town (Tabriz) |
54 (62.8%) |
| Other cities |
8 (9.3%) |
| Unknown |
24 (27.9%) |
| Covid-19 diagnostic method |
PCR |
70 (81.4%) |
| CT scan |
50 (58.1%) |
| Common covid-19 symptoms |
Fever |
67 (78.8%%) |
| Cough |
63 (74.1%) |
| Myalgia |
41 (48.2%) |
| Dyspnea |
30 (35.3%) |
| Anosmia |
22 (25.9%) |
| SPO2% |
< 90% |
34 (40%) |
| > 90% |
52 (60.5%) |
| Hospital admission for Covid-19 |
Yes |
39 (45.3%) |
| No |
47 (54.7%) |
| Sever COVID-19 |
Yes |
44 (51.2%) |
| No |
42 (48.8%) |
Dermatologic findings of patients
In general, 18 specific types of dermatologic complications were seen among studied patients (Table 2). The most common lesions included maculopapular rash (29.1%), scalp hair loss (29.1%), papular urticaria (8.1%), aphthous stomatitis (7%), erythematous macules (5.8%), lichenoid rashes (5.8%) and urticaria (5.8%) (Figure 1). Telogen effluvium was the only type of scalp hair loss in our patients. while maculopapular rash was significantly more common among male patients (16 vs. 9 cases, P = 0.008), the prevalence of telogen effluvium was higher in females (21 vs. 4, P = 0.002). No statistically significant difference regarding sex was seen in patients with other types of lesions (P > 0.05).
Table 2.
Features regarding dermatologic diagnoses of the patients
|
|
Total
(N=86)
|
Gender (n=86)*
|
Age
(Years, n=86)
|
Onset (n=78)*
|
Time of occurrence (days, n=78)
|
COVID-19 severity (n=86)
|
P
value**
|
Male
(n=36)
|
Female
(n=50)
|
Early
(n=48)
|
Late
(n=30)
|
Non-sever
(n=42)
|
Sever
(n=44)
|
| Maculopapular rash |
25 (29.1%) |
16 (44.4%) |
9 (18%) |
41.1 ± 20.9 |
20 (41.7%) |
2 (6.7%) |
2 (0-7) |
4 (9.5%) |
21 (47.7%) |
< 0.001 |
| Telogen effluvium |
25 (29.1%)*** |
3 (9.1%) |
20 (41.7%) |
38.5 ± 12.3 |
0 (0%) |
22 (73.3%) |
60 (45-78.7) |
19 (45.2%) |
6 (13.6%) |
0.001 |
| Papular urticaria |
7 (8.1%) |
3 (8.3%) |
4 (8%) |
41.7 ± 19.9 |
7 (14.6%) |
0 (0%) |
6.4 ± 5.6 |
4 (9.5%) |
3 (6.8%) |
0.710 |
| Oral aphthous ulcer |
6 (7.0%) |
2 (5.6%) |
4 (8%) |
41.3 ± 7.8 |
6 (12.5%) |
0 (0%) |
4.0 ± 5.2 |
2 (4.8%) |
4 (9.1%) |
0.677 |
| Red macules |
5 (5.8%) |
4 (9.7%) |
1 (2%) |
39.4 ± 19.0 |
3 (6.3%) |
0 (0%) |
1.6 ± 1.5 |
0 (0%) |
5 (11.4%) |
0.056 |
| Non-maculopapular rash |
5 (5.8%) |
2 (5.6%) |
3 (6%) |
28.8 ± 27.0 |
5 (10.4%) |
0 (0%) |
5.2 ± 2.8 |
1 (2.4%) |
4 (9.1%) |
0.361 |
| Urticaria |
5 (5.8%) |
2 (5.6%) |
3 (6%) |
26.2 ± 16.9 |
5 (10.4%) |
0 (0%) |
9.8 ± 2.6 |
5 (11.9%) |
0 (0%) |
0.024 |
| Hypopigmentation |
3 (3.5%) |
1 (2.8%) |
2 (4%) |
29.0 ± 21.0 |
1 (2.1%) |
2 (6.7%) |
18.0 ± 10.8 |
2 (4.8%) |
1 (2.3%) |
0.612 |
| Red patches |
3 (3.5%) |
2 (5.6%) |
1 (2%) |
8.6 ± 4.5 |
3 (6.3%) |
0 (0%) |
5.0 ± 0 |
0 (0%) |
3 (6.8%) |
0.242 |
| Oral lichenoid eruption |
2 (2.3%) |
0 (0%) |
2 (4%) |
49 (45-53) |
0 (0%) |
2 (6.7%) |
30.0 ± 0 |
2 (4.8%) |
0 (0%) |
0.236 |
| Vesicular eruption |
2 (2.3%) |
1 (2.8%) |
1 (2%) |
29 (8-50) |
1 (2.1%) |
1 (3.3%) |
14.5 (14-15) |
2 (4.8%) |
0 (0%) |
0.236 |
| Morbilliform rash |
2 (2.3%) |
1 (2.8%) |
1 (2%) |
34.5 (4-65) |
2 (4.2%) |
0 (0%) |
4 (3-5) |
1 (2.4%) |
1 (2.3%) |
1.000 |
| Scaling |
2 (2.3%) |
0 (0%) |
2 (4%) |
52 (27-77) |
1 (2.1%) |
1 (3.3%) |
17 (14-20) |
1 (2.4%) |
1 (2.3%) |
1.000 |
| Acneiform eruption |
1 (1.2%) |
0 (0%) |
1 (2%) |
31 ± 0 |
0 (0%) |
1 (3.3%) |
20 ± 0 |
1 (2.4%) |
0 (0%) |
0.488 |
| Livedo reticularis |
1 (1.2%) |
0 (0%) |
1 (2%) |
60.0 ± 0 |
0 (0%) |
1 (3.3%) |
21.0 ± 0 |
1 (2.4%) |
0 (0%) |
0.488 |
| Cyanosis on toes |
1 (1.2%) |
0 (0%) |
1 (2%) |
60.0 ± 0 |
0 (0%) |
1 (3.3%) |
21.0 ± 0 |
1 (2.4%) |
0 (0%) |
0.488 |
| Pustular psoriasis |
1 (1.2%) |
1 (2.8%) |
0 (0%) |
40.0 ± 0 |
1 (2.1%) |
0 (0%) |
8.0 ± 0 |
1 (2.4%) |
0 (0%) |
0.488 |
| Petechia/Purpura |
1 (1.2%) |
1 (2.8%) |
0 (0%) |
9.0 ± 0 |
1 (2.1%) |
0 (0%) |
8.0 ± 0 |
0 (0%) |
1 (2.3%) |
1.000 |
| Total number of lesions |
97 (100%) |
41 (42.3%) |
56 (57.7%) |
36.9 ± 17.7 |
56 (62.9%) |
33 (37.1%) |
9.5 (2-45) |
47 (48.4%) |
50 (53.6%) |
- |
Dermatologic manifestations appeared within first 14 days after first symptoms of COVID-19 were considered as < Early > lesions.
Dermatologic manifestations appeared after 14 days following first symptoms of COVID-19 were considered as < Late > lesions.
Quantitative data are shown as mean ± SD
Qualitative data are shown as frequency (%)
* Significantly (P < 0.05) different lesions regarding gender and onset time are shown in bold.
** The p-Values are calculated using Chi-squared or Fisher’s exact tests and show the significance level of differences between non-severe and severe COVID-19 infection.
*** There were 2 missing data of gender among patients with telogen effluvium.
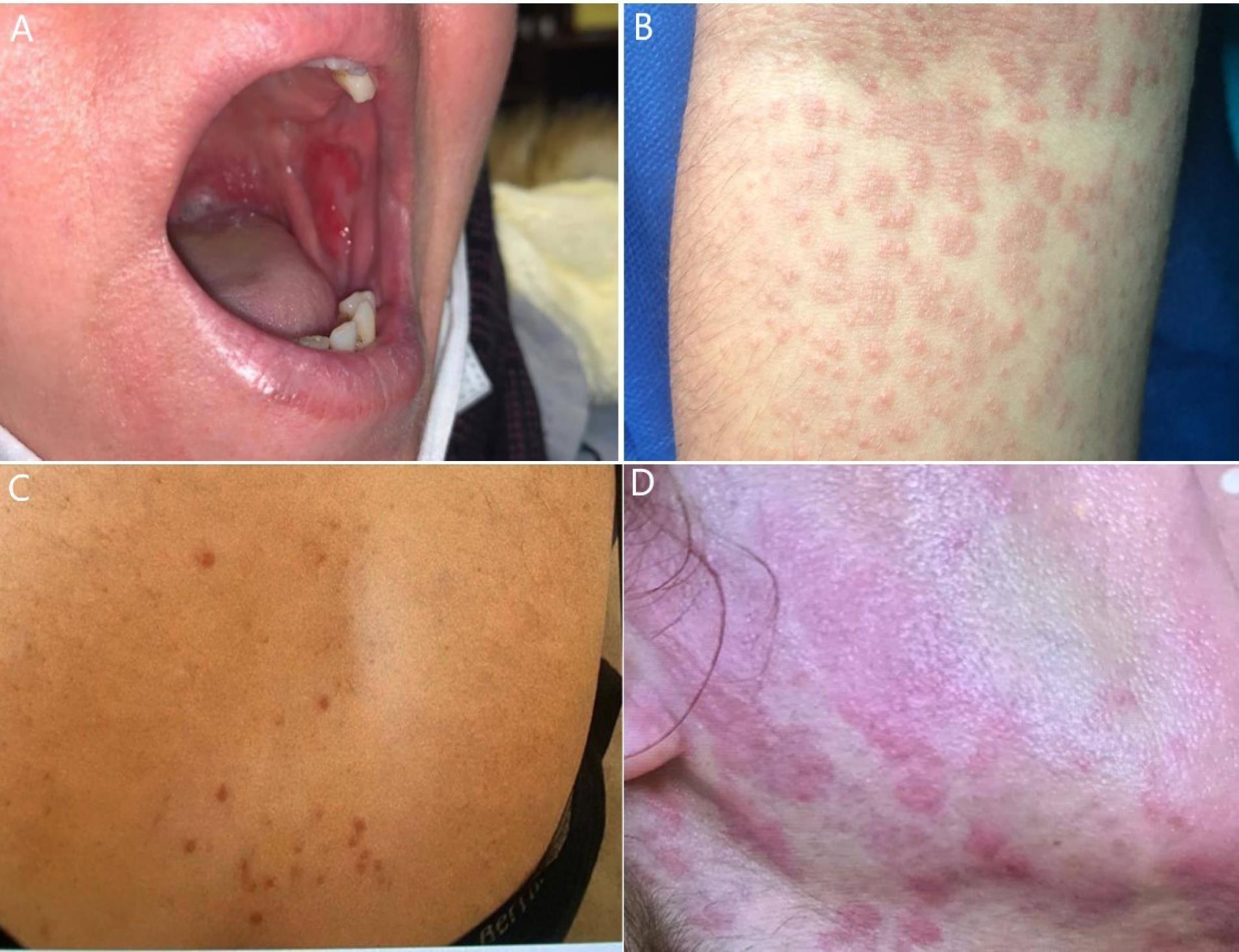
Figure 1.
Images of four skin lesions observed among our patients. (A) The patient was a 32-year-old female who manifested a painful aphthous ulcer on her left buccal side a weak after mild COVID-19 infection who was treated with Aphtin gel, (B) images of maculopapular rashes with mild pruritus on antecubital region of a 42-year-old male patient which manifested two weeks after severe COVID-19 infection and hospitalization. Although the only treatment included antipruritics, lesions were totally cured on patient’s follow-up (C) papular urticaria lesions on the region between two shoulders of a 25-year-old female patient who referred with pruritis symptom one week after mild COVID-19 infection. The patient was treated with antipruritics and antihistamine (D) Urticarial lesions on the face and neck region of a 30-year-old patient who referred to our clinic with pruritis symptom a week after mild COVID-19 infection. Patient’s therapies included antipruritics and antihistamine.
.
Images of four skin lesions observed among our patients. (A) The patient was a 32-year-old female who manifested a painful aphthous ulcer on her left buccal side a weak after mild COVID-19 infection who was treated with Aphtin gel, (B) images of maculopapular rashes with mild pruritus on antecubital region of a 42-year-old male patient which manifested two weeks after severe COVID-19 infection and hospitalization. Although the only treatment included antipruritics, lesions were totally cured on patient’s follow-up (C) papular urticaria lesions on the region between two shoulders of a 25-year-old female patient who referred with pruritis symptom one week after mild COVID-19 infection. The patient was treated with antipruritics and antihistamine (D) Urticarial lesions on the face and neck region of a 30-year-old patient who referred to our clinic with pruritis symptom a week after mild COVID-19 infection. Patient’s therapies included antipruritics and antihistamine.
As also shown in Table 2, less common complications were listed as hypopigmentation (3.5%), red patch (3.5%), oral lichenoid eruption (2.3%), vesicular lesion (2.3%), morbilliform rash (2.3%), scaling (2.3%), acneiform eruption (1.2%), livedo reticularis (1.2%), cyanosis on toes (1.2%) and pustular psoriasis (1.2%), and petechiae or purpura (1.2%). The average age of patients with each skin lesion is mentioned in (Table 2).
Early vs. late lesions
The onset time of lesions in eight patients was unclear. 48 out of 78 patients (61.5%) with 56 lesions (62.9%) had an early onset time (within 14 days after first symptom of COVID-19) The only lesions with statistically significant difference regarding onset time included maculopapular rash (P < 0.001), telogen effluvium (P < 0.001), papular urticaria (P = 0.039). While all patients with telogen effluvium were in the late-onset group, all cases with papular urticaria were in early-onset group. The vast majority of maculopapular rashes had an early onset (20 vs. 2). Moreover, all cases with oral aphthous ulcer, red macules, non-maculopapular rash, urticaria, morbilliform rash, pustular psoriasis, and petechia/purpura had an early onset (P > 0.05). On the other hand, all patients with oral lichenoid eruption, acneiform eruption, livedo reticularis, and cyanosis on toes were in in the late on-set group (P > 0.05). The median (IQR) of incidence time among all included lesions was 9.5 (2-45) days (Table 2).
Lesions in severe and non-severe COVID-19 patients
Based on the COVID-19 severity criteria, 42 (48.8%) patients had non-severe and 44 (51.2%) patients had severe COVID-19 infection (Table 2). The total number of lesions in non-severe and severe COVID-19 groups were 47 (48.4%) and 50 (53.6%), respectively. The only lesions with statistically significant difference regarding COVID-19 severity consisted of maculopapular rash, telogen effluvium, and urticaria. Maculopapular rash was more common among patients with severe COVID-19 (21 vs. 4, P < 0.001). Telogen effluvium was found in 19 patients with non-severe and 6 patients with severe COVID-19 (P = 0.001). All cases with urticaria had non-severe COVID-19 (P = 0.024). Conversely, all patients with red macules, red patches and petechia or purpura had severe COVID-19 (P > 0.05). None of the cases with severe COVID-19 had oral lichenoid eruption, vesicular eruption, acneiform eruption, livedo reticularis, cyanosis on toes, or pustular psoriasis (P > 0.05).
Skin involvement site and dermatologic symptoms of the patients
Skin involvement sites and dermatologic symptoms of the patients are shown in Table 3. Scalp involvement was seen in 26 (30.2%) cases, one of which was papular urticaria and the rest were telogen effluvium. Trunk (24.4%), upper limb (17.4%), back (11.6%), lower limb (7%), and face (5.8%) were the next most common skin sites of involvement. Mucosa was involved in 9 cases of patients (10.5%). The full body skin involvement was seen among 14 patients (16.3%). Although, the most frequent cutaneous symptoms were listed as pruritus (35.5%) and pain (10.6%), most of the patients were asymptomatic (55.3%). Six out of nine patients with pain symptom had oral aphthous ulcer, two had oral lichenoid eruption, and one had non-maculopapular rash.
Table 3.
Skin involvement site and dermatologic symptoms of the patients
|
Characteristic (N=86)
|
Frequency (%)
|
| Skin involvement site |
|
| Scalp |
26 (30.2%) |
| Trunk |
21 (24.4%) |
| Upper limb |
15 (17.4%) |
| Full body |
14 (16.3%) |
| Back |
10 (11.6%) |
| Mucosa |
9 (10.5%) |
| Lower limb |
6 (7.0%) |
| Face |
5 (5.8%) |
| Genitalia |
0 (0.0%) |
| Buttocks |
0 (0.0%) |
| Dermatologic symptoms |
|
| Pruritus |
30 (35.5%) |
| Pain |
9 (10.6%) |
| Hypoesthesia |
1 (1.2%) |
| Asymptomatic |
47 (55.3%) |
Discussion
SARS-CoV-2 has been responsible for millions of deaths around the globe since its breakout. Other than lungs, as the main impacted site in SARS-CoV-2 infection, many organs in human body including skin are affected by this virus. Along with the death tolls and hospitalizations surge, some reports regarding skin involvement following SARS-CoV-2 infection have drawn dermatologist’s notice. In this study we assessed dermatologic manifestations of patients with a history of recent COVID-19 infection. We further assessed the relationship between the severity of COVID-19 and the dermatologic manifestations presented in our dermatology clinics.
Overall, over the course of one year, we evaluated 86 patients with a mean age of 36.95 ± 17.78 years. Notably, the mean age in our study was lower compared to that in similar studies.11 This discrepancy could be due to higher severity of COVID-19 infection and mortality among elderly, difficulty in referring to a clinic or the higher importance of skin care among youth. Furthermore, most of our patients were female (50 vs. 36) which was in contrast to the systematic review study by Sachdeva et al.12 However, this finding may be attributable to the non-randomized nature of our study or could result from an underlying etiology. It has been demonstrated that androgens have a provoker effect on TMPRSS2, as the SARS-COV-2 importing factor, which may explain the higher prevalence of COVID-19 skin complications in male.13
Eighteen types of skin lesions were detected in our patients, among which maculopapular rashes (29.1%), telogen effluvium (29.1%), popular urticaria (8.1%), oral aphthous ulcers (7%) and red macules (5.8%) were the most common ones.
In most cases (55.3%), the skin complication caused no discomfort for the patients and they were asymptomatic. However, the most prevalent symptoms were pruritus (35.5%) and pain (10.6%). In another study conducted by Li et al, results revealed a prevalence of 55.3% for itching and 12.9% for symptom of pain.14
In our study we found that the scalp was the most commonly affected site, with a prevalence of 30.2%. Specifically, we observed a high prevalence of telogen effluvium type of involvement, accounting for 29.1% of cases. The trunk and upper limbs followed as the second and third most affected sites with a prevalence of 24.4% and 17.4%, respectively. In contrast, a systematic review conducted by Schwartzberg et al, which included 42 articles, reported the trunk as the most commonly involved site (37.4%) followed by the lower limbs (16%).15 A study by Li et al, revealed similar results and the trunk and lower limbs were the first and second most affected areas (51.4% and 31.7% respectively).14 The difference between the results of our study and those of these articles may be due to the inclusion of telogen effluvium as a dermatologic manifestation, which was not included in other studies.
Maculopapular rashes
Large eruption areas with red, tiny, confluent bumps are a common description of maculopapular eruptions.16 maculopapular rash has been the most common COVID-19 dermatologic complication in several studies.12,17-19 In our study, the most frequent dermatological complication observed was a maculopapular rash as well, accounting for 29.1% of cases. This finding aligns with a study conducted by Li et al, where the maculopapular rash was also reported as the most common type of skin involvement. However, in Schwartzberg and colleagues’ study,15 pernio-like lesions had a prevalence of 16.56%, followed by morbilliform lesions (rose-colored macules and papules) with a prevalence of 13.5%. In our assessment, maculopapular rashes were more common among males, with a prevalence of 44.4%, compared to 18% in females. This could potentially be attributed to hormonal factors and the role of the immune system, specifically the pro-inflammatory effects of testosterone and the anti-inflammatory effects of estrogen. Majority of our cases experienced these lesions within first 14 days after first symptoms of COVID-19 (early-onset). Additionally, we found that individuals with severe COVID-19 were more likely to develop maculopapular rashes (21 severe compared to 4 non-severe cases). In the systematic review by Jamshidi et al, which included 47 articles and 1847 patients, the most prevalent skin manifestation was maculopapular rashes. These were associated with intermediate severity of COVID-19 infection.7 In another review of 240 papers, patients presenting with acro-ischemia, purpura, livedo reticularis, urticaria, maculopapular rash, erythema, and changes in hair and nails, as well as oral ulcers, had a higher likelihood of hospital admission during the course of their COVID-19 illness.20 These findings suggest a potential association between the severity of COVID-19 and the manifestation of maculopapular rashes. One possible explanation is that the rashes are caused by a hyperactive immune response, possibly due to cytokine storm and overactivation of the immune system in severe cases, making these manifestations more common.
Telogen effluvium (TE)
Telogen effluvium is characterized as a non-inflammatory phenomenon that manifests several months after a physiological insult, such as severe illness, affects the body. This event induces a transition of anagen hair follicles into the telogen phase, culminating in marked and sudden hair loss.21 Telogen effluvium, as the other most common skin lesion (29.1%), was late-onset in all affected patients (average onset-time = 60 days) similar to the study by Olds et al on 552 patients at Henry Ford hospital.22 Telogen effluvium was more common among females (20:3 P = 0.001). In a study conducted by Seyfi et al., which included 198 COVID-19 patients, 60.1% were female; however, the p-value was 0.51, indicating no statistical significance.23 In another study examining 47 patients experiencing acute hair loss post-COVID-19 infection, the gender distribution was 27.6% male and 72.4% female.24 Hair loss was notably more prevalent in cases of non-severe COVID-19. (19:6, P = 0.001). However, controversies regarding severity of COVID-19 and prevalence of telogen effluvium are seen in different studies. In a study performed on a small sample of patients with telogen effluvium, majority of cases (6 of 10) had mild COVID-19 infection.25 On the other hand, seven out of ten telogen effluvium cases in another research had a history of severe COVID-19 infection, same as our study.22 Additionally, in a research by Aksoy et al, among 57 cases of telogen effluvium associated with COVID-19, TE was more frequent in hospitalized patients compared to outpatients, although the difference was not statistically significant (31.7% vs. 24.3%; P =0.238).26 The patients who presented to our clinic had already been discharged from the hospital, suggesting that we may have primarily encountered cases of telogen effluvium associated with mild COVID-19. Further studies are required to adequately assess the relationship between COVID-19 severity and telogen effluvium.
Urticaria
Urticaria is defined as the presence of erythematous plaques and papules that are pruritic and transient, originating from mast cell activation.27 We detected seven cases of papular urticaria (8.1%). All of these lesions appeared within the two weeks following the first COVID-19 symptom (Mean = 6.4 days). We also diagnosed urticarial lesions in five cases (7%) with an average onset time of 9.8 days, all of which experienced a non-severe COVID-19 (P = 0.024). In a review of 47 articles that included 1847 patients, urticaria-like lesions in COVID-19 patients were correlated with a lower mortality rate of 2.2%. Patients with these lesions generally experienced milder disease severity, a finding that is concordant with our results.7 In another review conducted by Algaadi, 30 articles featuring a total of 202 patients with COVID-19-associated urticaria were analyzed. The level of care was reported for 129 patients (64%); among these, 14 (11%) required treatment in the intensive care unit (ICU), while 115 (89%) received standard care either as outpatients or inpatients.28
Mucosal involvement
Patients with more severe manifestations of oral mucosal disease are at elevated risk for experiencing a severe form of COVID-19 infection.29 Oral aphthous ulcers are one of the frequently diagnosed lesions among COVID-19 patients30 and are defined as well-demarcated, superficial ulcerative lesions, distinguished by a white periphery and a white or yellow pseudomembrane. These lesions are notably associated with significant pain.31 Six of our patients experienced painful oral aphthous ulcers (7%) which averagely appeared within four days after COVID-19 infection (during active infection). The mean age of affected cases was 49 years and 66% of them were females. In a systematic review conducted by Swain et al, non-necrotic aphthous ulcers were predominantly observed among younger patients and those with mild COVID-19 infection. Conversely, necrotic ulcers were more frequently encountered in patients with severe COVID-19 infection.32
Limitations
Relatively small population size along with non-probability sampling methods were the main limitations of our study. We also did not have access to the hospitalization or COVID-19 test documents of most patients and these information were collected based on their own statements which may cause study bias. Therefore, more systematic and larger samples are suggested for future studies.
Study Highlights
What is current knowledge?
-
The dermatologic manifestations associated with COVID-19 have been reported since the early stages of the pandemic. These manifestations are believed to be linked to the endothelial dysfunction and inflammatory responses triggered by the virus. Commonly observed skin lesions include maculopapular rashes, pseudo-chilblain and vesicular lesions which have been noted to correlate with the severity of the disease. Previous studies have documented a prevalence of these manifestations and their potential as indicators of systemic involvement or disease severity
What is new here?
-
This study contributes new insights by providing a comprehensive analysis of dermatologic manifestations in a specific regional population, focusing on the correlation between the severity of COVID-19 and the occurrence of specific skin lesions. The findings highlight the statistically significant association of maculopapular rashes, telogen effluvium, and urticaria with COVID-19 severity. Moreover, the study uniquely documents the timing of lesion onset relative to the disease course, distinguishing between early and late dermatologic manifestations and providing valuable data for clinical practice in similar settings.
Conclusion
Although COVID-19 infection most frequently presents with respiratory manifestations, skin involvement in these patients is not uncommon. The possible underlying mechanisms for such lesions are diffuse microvascular thrombosis and viral exanthem. the wide spectrum of skin lesions among our patients included maculopapular rash, telogen effluvium, and papular urticaria. Given the broad range of dermatologic manifestations associated with COVID-19, it is crucial for clinicians to remain vigilant in identifying and managing these symptoms to improve patient outcomes and guide further research into their underlying mechanisms.
Acknowledgments
We would like to express our gratitude to Dr. Morteza G. for his invaluable assistance in analysis of our data.
Competing Interests
The authors declare no conflicts of interest regarding this study. They have not received any financial support or compensation related to this research.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Restrictions apply to the availability of these data, which were used under license for this study. Data are not publicly available due to privacy or ethical restrictions. The corresponding author can be contacted for any inquiries regarding the data sharing statement.
Ethical Approval
This study strictly adhered to ethical guidelines and obtained written informed consent from all participants or their legal guardians. Patients’ confidentiality was guaranteed through data anonymization, and all recognizable information was securely stored. The study was approved by the Tabriz University of Medical Sciences ethics committee (IR.TBZMED.REC.1400.558). The research team maintained data integrity through standardized data collection and independent review of dermatologic manifestations. Patient images were used with consent, and measures were taken to protect their identities.
References
- World Health Organization (WHO). WHO Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. WHO; 2020.
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus A first step in understanding SARS pathogenesis. J Pathol 2004; 203(2):631-7. doi: 10.1002/path.1570 [Crossref] [ Google Scholar]
- Hejazian SS, Hejazian SM, Farnood F, Abedi Azar S. Dysregulation of immunity in COVID-19 and SLE. Inflammopharmacology 2022; 30(5):1517-31. doi: 10.1007/s10787-022-01047-2 [Crossref] [ Google Scholar]
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395(10234):1417-8. doi: 10.1016/s0140-6736(20)30937-5 [Crossref] [ Google Scholar]
- Bouaziz JD, Duong TA, Jachiet M, Velter C, Lestang P, Cassius C. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatol Venereol 2020; 34(9):e451-2. doi: 10.1111/jdv.16544 [Crossref] [ Google Scholar]
- Tahavvori A, Mosaddeghi-Heris R, Ghanbari Sevari F, Alavi SM, Panahi P, Abbasi N. Combined systemic inflammatory indexes as reflectors of outcome in patients with COVID-19 infection admitted to ICU. Inflammopharmacology 2023; 31(5):2337-48. doi: 10.1007/s10787-023-01308-8 [Crossref] [ Google Scholar]
- Jamshidi P, Hajikhani B, Mirsaeidi M, Vahidnezhad H, Dadashi M, Nasiri MJ. Skin manifestations in COVID-19 patients: are they indicators for disease severity? A systematic review. Front Med (Lausanne) 2021; 8:634208. doi: 10.3389/fmed.2021.634208 [Crossref] [ Google Scholar]
- Seque CA, Enokihara M, Porro AM, Tomimori J. Skin manifestations associated with COVID-19. An Bras Dermatol 2022; 97(1):75-88. doi: 10.1016/j.abd.2021.08.002 [Crossref] [ Google Scholar]
- Sameni F, Hajikhani B, Yaslianifard S, Goudarzi M, Owlia P, Nasiri MJ. COVID-19 and skin manifestations: an overview of case reports/case series and meta-analysis of prevalence studies. Front Med (Lausanne) 2020; 7:573188. doi: 10.3389/fmed.2020.573188 [Crossref] [ Google Scholar]
- Sharma AN, Mesinkovska NA, Paravar T. Characterizing the adverse dermatologic effects of hydroxychloroquine: a systematic review. J Am Acad Dermatol 2020; 83(2):563-78. doi: 10.1016/j.jaad.2020.04.024 [Crossref] [ Google Scholar]
- Gallo Marin B, Aghagoli G, Lavine K, Yang L, Siff EJ, Chiang SS. Predictors of COVID-19 severity: a literature review. Rev Med Virol 2021; 31(1):1-10. doi: 10.1002/rmv.2146 [Crossref] [ Google Scholar]
- Sachdeva M, Gianotti R, Shah M, Bradanini L, Tosi D, Veraldi S. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci 2020; 98(2):75-81. doi: 10.1016/j.jdermsci.2020.04.011 [Crossref] [ Google Scholar]
- Lin B, Ferguson C, White JT, Wang S, Vessella R, True LD. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res 1999; 59(17):4180-4. [ Google Scholar]
- Li H, Zhao Y, Zhou L, Hu J. Cutaneous, skin histopathological manifestations and relationship to COVID-19 infection patients. Dermatol Ther 2020; 33(6):e14157. doi: 10.1111/dth.14157 [Crossref] [ Google Scholar]
- Schwartzberg LN, Advani S, Clancy DC, Lin A, Jorizzo JL. A systematic review of dermatologic manifestations among adult patients with COVID-19 diagnosis. Skin Health Dis 2021; 1(2):e20. doi: 10.1002/ski2.20 [Crossref] [ Google Scholar]
- Yap EW. Rash, red eyes, lip erosions and genital ulcer - what is the diagnosis?. Malays Fam Physician 2022; 17(1):99-102. doi: 10.51866/tyk1371 [Crossref] [ Google Scholar]
- Galván Casas C, Català A, Carretero Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, Rodríguez-Villa Lario A. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183(1):71-7. doi: 10.1111/bjd.19163 [Crossref] [ Google Scholar]
- Dalal A, Jakhar D, Agarwal V, Beniwal R. Dermatological findings in SARS-CoV-2 positive patients: an observational study from North India. Dermatol Ther 2020; 33(6):e13849. doi: 10.1111/dth.13849 [Crossref] [ Google Scholar]
- Ocampo-Candiani J, Ramos-Cavazos CJ, Arellano-Mendoza MI, Arenas-Guzmán R, Beirana-Palencia A, Salmon-Demongin A. International registry of dermatological manifestations secondary to COVID-19 infection in 347 Hispanic patients from 25 countries. Int J Dermatol 2021; 60(8):956-63. doi: 10.1111/ijd.15632 [Crossref] [ Google Scholar]
- Holmes Z, Courtney A, Lincoln M, Weller R. Rash morphology as a predictor of COVID-19 severity: a systematic review of the cutaneous manifestations of COVID-19. Skin Health Dis 2022; 2(3):e120. doi: 10.1002/ski2.120 [Crossref] [ Google Scholar]
- Ozlu E, Karadağ AS. Telogen effluvium. In: Hair and Scalp Disorders. IntechOpen; 2017. 10.5772/66975.
- Olds H, Liu J, Luk K, Lim HW, Ozog D, Rambhatla PV. Telogen effluvium associated with COVID-19 infection. Dermatol Ther 2021; 34(2):e14761. doi: 10.1111/dth.14761 [Crossref] [ Google Scholar]
- Seyfi S, Alijanpour R, Aryanian Z, Ezoji K, Mahmoudi M. Prevalence of telogen effluvium hair loss in COVID-19 patients and its relationship with disease severity. J Med Life 2022; 15(5):631-4. doi: 10.25122/jml-2021-0380 [Crossref] [ Google Scholar]
- Kant T, Narain U, Kant A. Acute telogen effluvium: a sequela of COVID-19 infection. Glob J Res Anal 2022; 11(5):114-5. [ Google Scholar]
- Mieczkowska K, Deutsch A, Borok J, Guzman AK, Fruchter R, Patel P. Telogen effluvium: a sequela of COVID-19. Int J Dermatol 2021; 60(1):122-4. doi: 10.1111/ijd.15313 [Crossref] [ Google Scholar]
- Aksoy H, Yıldırım UM, Ergen P, Gürel MS. COVID-19 induced telogen effluvium. Dermatol Ther 2021; 34(6):e15175. doi: 10.1111/dth.15175 [Crossref] [ Google Scholar]
- Schettini N, Corazza M, Schenetti C, Pacetti L, Borghi A. Urticaria: a narrative overview of differential diagnosis. Biomedicines 2023; 11(4):1096. doi: 10.3390/biomedicines11041096 [Crossref] [ Google Scholar]
- Algaadi SA. Urticaria and COVID‐19: a review. Dermatol Ther 2020; 33(6):e14290. doi: 10.1111/dth.14290 [Crossref] [ Google Scholar]
- Doceda MV, Gavriiloglou M, Petit C, Huck O. Oral health implications of SARS-CoV-2/COVID-19: a systematic review. Oral Health Prev Dent 2022; 20:207-18. doi: 10.3290/j.ohpd.b2960801 [Crossref] [ Google Scholar]
- Fidan V, Koyuncu H, Akin O. Oral lesions in COVID-19 positive patients. Am J Otolaryngol 2021; 42(3):102905. doi: 10.1016/j.amjoto.2021.102905 [Crossref] [ Google Scholar]
- Iranmanesh B, Khalili M, Amiri R, Zartab H, Aflatoonian M. Oral manifestations of COVID-19 disease: a review article. Dermatol Ther 2021; 34(1):e14578. doi: 10.1111/dth.14578 [Crossref] [ Google Scholar]
- Swain SK, Debta P, Sahu A, Lenka S. Oral cavity manifestations by COVID-19 infections: a review. Int J Otorhinolaryngol Head Neck Surg 2021; 7(8):1391-7. doi: 10.18203/issn.2454-5929.ijohns20212914 [Crossref] [ Google Scholar]