J Res Clin Med. 12:39.
doi: 10.34172/jrcm.34476
Original Article
Aerobic exercise combined with whole body vibration in non-alcoholic fatty liver disease: A randomized controlled trial
Yaghoub Salekzamani Conceptualization, Methodology, 1 
Manouchehr Khoshbaten Supervision, Writing – review & editing, 2
Azizeh Farshbaf-Khalili Formal analysis, Investigation, 1
Soraya Babaie Data curation, Writing – original draft, 1, * 
Author information:
1Physical Medicine and Rehabilitation Research Center, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
2Liver and Gastrointestinal Disease Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
A whole-body vibration device (WBV) as an exercise option may be suitable for nonalcoholic fatty liver disease (NAFLD). This study aimed to compare the effects of combined aerobic exercise with WBV training and aerobic exercise alone on the liver enzymes and lipid profiles in NAFLD.
Methods:
This randomized clinical trial was conducted on 32 patients with grade 1 and 2 NAFLD diagnosed by ultrasound. Patients were randomly divided into intervention and control groups. Patients in the intervention group were subjected to WBV with a frequency of 35-50 Hz before starting aerobic exercise. Wherein, aerobic exercises were performed using a treadmill at 60%-80% of maximum heart rate. Each session included a 5-minute warm-up, 30 minutes of treadmill, and 5 minutes of cooling at the end. Subjects in the control group underwent the same protocol but did not undergo WBV. Sessions were performed three times per week. The intervention lasted for eight weeks for both groups. Liver enzymes (alkaline phosphatase [ALP], alanine aminotransferase [ALT], aspartate aminotransferase [AST]), and lipid profiles (high-density lipoprotein [HDL], low-density lipoprotein [LDL], triglyceride, and cholesterol) were measured during a week before the exercises and after the trial.
Results:
Mean serum ALT levels (45.5 vs. 31.5; P=0.003) and triglycerides (226.7 vs. 209.3; P=0.004) levels significantly decreased in the intervention group after the intervention. Although all liver enzymes and lipid profiles serum levels except for LDL decreased significantly in both groups (P<0.05), AST, ALP, cholesterol, LDL, and HDL levels showed no significant differences between the two groups.
Conclusion:
The present study revealed that aerobic exercises with WBV significantly reduced ALT and triglyceride levels compared to aerobic exercises alone. In conclusion, the combined method can be recommended as a more effective method for patients suffering from NAFLD.
Keywords: Aerobic, Non-alcoholic fatty liver disease, Vibration
Copyright and License Information
© 2024 The Authors.
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
There is no funding support to conduct the research.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a complex liver disease that follows a progressive course, with various stages from the initial accumulation of fat in the liver to the development of nonalcoholic steatohepatitis, ultimately leading to liver cirrhosis and hepatocellular carcinoma.1 The etiology of NAFLD remains poorly understood, with estimated differences observed among individuals. Nevertheless, the “multiple-hit hypothesis” serves as a valuable framework to enhance our comprehension of this condition. The initial occurrence of hepatic steatosis can be regarded as the “first hit”, while subsequent or concurrent combinations of genetic predispositions, metabolic dysfunction (mainly insulin resistance), lifestyle factors, dietary patterns, and/or changes in the gut microbiome are all potential factors that may contribute to the progression of more severe liver disorders.2 The activation of hepatic stellate cells and Kupffer cells is facilitated by the sequential or simultaneous activation of the adaptive and innate immune systems, leading to the progression of fibrosis and cirrhosis.3,4 Oftentimes, elevated transaminases are discovered during routine laboratory tests or when evaluating other disorders such as hypertension, hyperlipidemia, obesity, and diabetes.5 Elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are the most common laboratory abnormality. There is rarely an elevation exceeding 2 to 3 times the upper limits of normal.6 The diagnosis of NAFLD is subject to several criteria. There must be no history of significant alcohol use among the patients under consideration. The management of glucolipid metabolism remains a primary focus in the treatment of NAFLD. Furthermore, there has been an increase in research efforts focused on the manipulation of the gut microbiota as a potential approach for the treatment of NAFLD. Pharmaceutical interventions have not been successful in treating NAFLD, and researchers are currently developing various medications that are undergoing clinical trials.7 Therefore, lifestyle intervention involving dietary changes and structured exercise is the mainstay of treatment.8 Developing NAFLD is arguably influenced most by weight gain, whereas weight loss leads to decreased liver fat and aminotransferase levels. It has been established that physical exercise is beneficial for managing NAFLD.9 However, it may be difficult for some people to maintain exercise in daily life without assistance. Patients who have difficulty engaging in exercise may benefit from whole-body vibration device (WBV) as an exercise modality.10 Unlike pharmacotherapies, exercise is the foundation of NAFLD management.11 A variety of health consequences are improved in patients with NAFLD through both aerobic and resistance exercise. Nevertheless, WBV is a novel alternative that has recently been introduced. By generating intense stimulation passively, this exercise changes the length of muscle fibers dynamically.10 During the procedure, patients are exposed to rapid and repeated oscillations via a vibration device. With brief exposure to vibration, this form of exercise stimulates muscles strongly. In various fields of medicine and physiology, WBV has been found to be effective. WBV induces physiological improvements in human subjects, impacting their neuromuscular, respiratory, and cardiovascular functions.12 The beneficial effects of this treatment have been studied and confirmed in various conditions, such as osteoporosis, fractures, and metabolic syndrome.13-15 Because of its effectiveness and ease of use, WBV is a suitable exercise method for patients who have difficulty engaging in general exercise.16,17 The interest in the WBV intervention arises from the fact that it requires minimal energy and motivation from the practitioner. In addition, it needs a minimal duration of exposure (approximately 5-15 minutes per session), rendering it an attractive alternative when the performance of normal physical exercises is impossible.18,19 The current study evaluated whether WBV in combination with aerobic exercise could reduce lipid profiles and liver enzymes more effectively than aerobic exercise alone in patients with NAFLD.
Materials and Methods
Study design and setting
This randomized clinical trial was conducted on 32 patients aged 27-62 years with grade 1 and 2 NAFLD diagnosed by ultrasound and referred to Imam Reza hospital in Tabriz. The process of participant recruitment up to the conclusion of the intervention lasted for a period of 18 months. Before starting the study, informed consent was obtained from all participants. The inclusion criteria were grade 1 and 2 NAFLD diagnosed by ultrasound and BMI between 27-35, the exclusion criteria were consumption of alcohol exceeding five units per week, the use of hepatotoxic drugs such as amiodarone, tamoxifen, methotrexate, fibrate, etc., and exercise contraindications such as cardiopulmonary or musculoskeletal conditions, including severe knee arthritis.
Randomization and intervention
Participants were randomly assigned to intervention groups (16 subjects) and control groups (16 subjects). Wherein, block randomization with fixed size was used for randomization. Because of the study type, it was not possible to blind therapists and patients but blinding was used during evaluation and analysis. In the convenience sampling method, gastroenterologists introduced samples as a series and then entered them into the study. A gastroenterologist diagnosed patients with NAFLD grade 1 or 2, and based on inclusion criteria, patients were included in this study. Patients in the intervention group were subjected to WBV (Azimuth standing massager and vibrator model AZ 904-1) with a frequency of 35-50 Hz before starting aerobic exercise. Wherein, aerobic exercises were performed using a treadmill at 60%-8% of maximum heart rate. Hence, each session included a 5-minute warm-up, 30 minutes of treadmill, and 5 minutes of cooling at the end. Subjects in the control group underwent the same protocol but did not undergo WBV. Sessions were performed 3 times per week. The intervention for both groups lasted for eight weeks. All interventions in two groups were conducted with the presence and supervision of the researcher, and therefore, except for two cases of sample loss, all participants had full participation in the entire procedure. In both groups, liver enzymes (alkaline phosphatase [ALP], ALT, AST) and lipid profiles (high-density lipoprotein [HDL], low-density lipoprotein [LDL], triglyceride, and cholesterol) were measured during a week before the exercises and after the trial. It was advised to all participants to continue their drug treatments and diet regimen during the study.
Sample size
We calculated the sample size based on the study of Khaoshbaten et al20 using G*Power software and taking into account m1 = 176, sd1 = 5.2 (the mean serum triglyceride after aerobic exercise) and m2 = 158.4, sd2 = sd1 = 5.2 (the mean serum triglyceride with an assumption of 10% decrement following adding WBV) α = 0.05, power = 80%, and two-sided test equal to 5 people in each group. Also, considering m1 = 43.5, sd1 = 2.4 (the mean serum ALT after aerobic exercise) and m2 = 39.15, sd2 = sd1 = 2.4 (the mean serum ALT with the assumption of 10% decrement following adding WBV), α = 0.05, power = 80%, and two-sided test equal to 15 people in each group. Finally, after considering the 10% possible dropout, 16 people for each group and a total of 32 people were estimated.
Statistical Analysis
In this study, before using statistical tests and making inferences based on the data, the distributions of cholesterol, triglyceride, AST, ALT, HDL, LDL, and ALP were evaluated before and after aerobic exercise by using the Kolmogorov-Smirnov test. To compare baseline variables, an independent t-test and Fisher’s exact test were used. Comparing before and after was performed using paired t-test and Wilcoxon, and laboratory values were compared using ANCOVA adjusted for baseline and Mann-Whitney. Depending on the type of hypothesis, appropriate statistical tests were performed and interpreted as one-tailed. All analysis was done using Stata analyses, and a p-value less than 0.05 was considered significant.
Results
The quantitative variables included in the current study were age, cholesterol, triglycerides, AST, ALT, HDL, LDL, and ALP. ALT and AST variables had non-normal distributions because of P values less than 0.05. Except for age, all of the variables were measured one week before and after the intervention. A total of 32 subjects participated in the study. Subjects were randomly assigned to the intervention group (16 subjects) and the control group (16 subjects). Within two weeks, one patient from each group was removed and 15 patients remained. The remaining 30 patients were 11 men (37%) and 19 women (63%) in total (Figure 1).
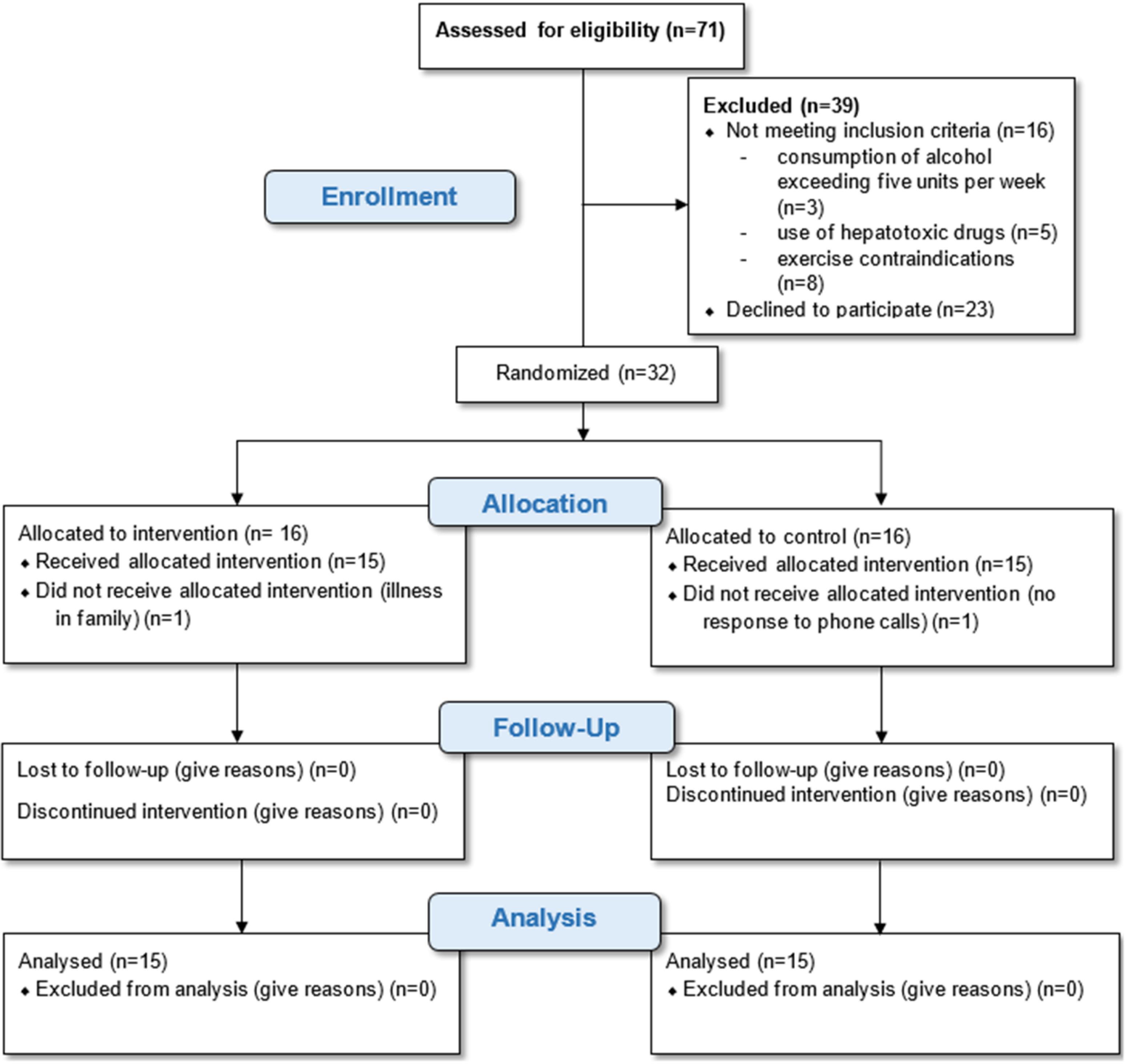
Figure 1.
The consort flowchart of study
.
The consort flowchart of study
In the control group, the average age was 41.35, while in the intervention group, it was 44.56 (P = 0.36). There were no significant differences between-groups in terms of BMI and gender (Table 1).
Table 1.
Baseline characteristics of the participants
|
Variables
|
Intervention
|
Control
|
P
value
|
| NAFLD, No. (%) |
Grade I |
10 (62.5) |
7 (43.7) |
0.479a |
| Grade II |
6 (37.5) |
9 (56.3) |
| Gender, No. (%) |
Male |
6 (37.5) |
5 (31.2) |
0.922a |
| Female |
10 (62.5) |
11(68.8) |
| Age (y), Mean ± SD |
44.56 ± 12.2 |
41.35 ± 13.5 |
0.362b |
| Body mass index (kg/m2), Mean ± SD |
28.8 ± 1.5 |
29.3 ± 1.9 |
0.536b |
NAFLD: Non-alcoholic fatty liver disease, SD: Standard deviation.
a Fisher exact test; b Independent t-test.
Liver enzyme levels in response to aerobic exercise with and without WBV
The mean ALT levels decreased significantly after aerobic exercises with and without vibration compared to baseline (P < 0.05). The difference between the two groups was significant (P = 0.003), indicating that aerobic exercise with vibration significantly decreased ALT in comparison with the control group (Table 2).
Table 2.
The comparison of basic information description before and after trial
|
Variable
|
Intervention
|
P
valuea
|
Control
|
P
valuea
|
P
valueb
|
|
Pre-test
|
Post-test
|
Pre-test
|
Post-test
|
| ALP |
133.0 ± 31.5 |
113.2 ± 22.3 |
<0.001
|
135.0 ± 33.6 |
123.5 ± 16.1 |
<0.001
|
0.064 |
| ALT |
47.3 ± 21.3 |
31.5 ± 15.1 |
<0.001
|
49.3 ± 19.5 |
45.5 ± 19.8 |
0.032
|
0.003
c
|
| AST |
48.4 ± 18.7 |
35.7 ± 13.7 |
<0.001
|
48.8 ± 16.8 |
41.5 ± 14.6 |
0.014
|
0.090c |
| Cholesterol |
234.9 ± 28.6 |
220.4 ± 22.4 |
<0.001
|
236.8 ± 31.6 |
225.8 ± 25.6 |
0.005
|
0.519 |
| Triglyceride |
243.7 ± 33.4 |
209.3 ± 18.3 |
<0.001
|
239.2 ± 37.5 |
226.7 ± 24.2 |
0.018
|
0.040
|
| LDL |
117.4 ± 17.9 |
112.5 ± 15.4 |
0.122 |
121.4 ± 17.9 |
116 ± 21.2 |
0.104 |
0.430 |
| HDL |
36.0 ± 5.5 |
39.7 ± 5.8 |
0.044
|
37.0 ± 5.5 |
40.1 ± 6.6 |
0.046
|
0.529 |
Abbreviations: ALT, alanine aminotransferase; AST, Aspartate aminotransferase; ALP, alkaline phosphatase; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Note: Total data are expressed as mean ± SD, with P values indicating statistical significance (P < 0.05) presented in bold.
aPaired t-test; bANCOA adjusted for baseline values; cMann-Whitney test.
The mean AST levels significantly decreased within both groups compared to baseline (p-value < 0.05). However, no significant between groups difference was observed following the intervention (P = 0.090; Table 2).
Both aerobic exercises with vibration and aerobic exercise alone reduced the mean serum ALP (P < 0.05). The comparison of ALP levels showed no significant difference between the intervention and control groups (P = 0.064; Table 2).
Lipid profile levels in response to aerobic exercise with and without WBV
The mean cholesterol levels significantly decreased within both groups compared to baseline (P < 0.05). However, no significant difference between groups was observed following intervention (P = 0.519; Table 2).
The mean triglyceride levels in aerobic exercises with vibration and aerobic exercise alone significantly decreased compared to baseline (P < 0.05). On the other hand, the comparison of triglyceride levels between the intervention and control groups displayed a significant difference (P = 0.04; Table 2).
In both the intervention and control groups, the mean LDL levels before and after aerobic exercise were not significantly different from baseline. However, no significant between groups difference was observed following intervention (P = 0.436; Table 2).
In both groups, aerobic exercises (whether vibrating or not vibrating) significantly decreased HDL levels compared to baseline (P < 0.05) in patients with NAFLD. Meanwhile, the comparison of HDL levels between the intervention and control groups displayed no significant difference (P = 0.829; Table 2).
Discussion
This study showed that WBV coupled with aerobic exercise reduced liver enzymes and fat levels in patients with NAFLD. Among 32 patients with fatty liver, patients undergoing WBV and aerobic exercise had lower levels of ALT and triglycerides than patients who performed aerobic exercise alone. However, it did not show any additional effect on LDL, cholesterol, HDL, AST, and ALP, although according to the findings of this study, in both groups, the levels of HDL, ALT, AST ALP, cholesterol, and triglycerides decreased significantly, but the level of LDL in neither group was reduced.
NAFLD is a multifaceted condition connected to diverse metabolic risk factors and increases the risk of mortality associated with hepatic and cardiovascular conditions. With the increase in life expectancy, NAFLD has emerged as a significant global health challenge. It has been well established that physical exercise is beneficial for managing NAFLD.21 A progressive loss of muscle mass and strength (sarcopenia) in older people, decreases the quality of life, increases the possibility of disability, and contributes to higher body fat, raising the risk of type 2 diabetes. Keeping active and participating in regular sports activities can delay the aging process, but there are risks associated with injuries and reduced mobility for the elderly.22 WBV is a safe method and can be used for a long time as a passive exercise modality, and it can directly increase energy consumption and thus cause weight loss, as evidenced in several studies. When combined with other treatment methods, such as diet restrictions or resistance exercises, this method can cause weight loss as well. Compared to young people, WBV may have more beneficial effects on elderly people, and it can cause further improvements in health and quality of life.23 According to the previous study that was conducted on the effect of aerobic exercise on NAFLD,24 short-term aerobic exercise increases the unsaturated fat indices, and similar results were observed in our study. However, the mentioned study found that aerobic exercise did not reduce triglyceride levels, whereas our study found that aerobic exercise also reduced triglyceride levels, most likely because the duration of exercise in our study was longer, and it appears that long-term aerobic exercise for at least eight weeks has a positive effect on triglycerides. According to another study in South Korea on the effects of regular aerobic exercise on non-alcoholic fatty liver patients,25 the patients were at reduced risk of developing NAFLD if these regular aerobic exercises were performed three times a week for a minimum of 30 minutes a day for more than three months. Additionally, it improves the metabolism of liver fat and liver enzymes, which we have also observed in our study to decrease fat and liver enzymes. Similarly, other studies have also reported a decrease in liver enzymes in non-alcoholic fatty liver patients following exercise.20 According to another study, WBV reduced visceral fat and liver triglyceride content in old rats in an animal study.23 Furthermore, the content of liver fat (9.9%) and visceral fat (6.2%) decreased significantly in alcoholic fatty liver patients following WBV therapy over 6 months in human samples.10 According to the results of this study, WBV combined with regular exercise reduces blood triglyceride levels and regulates liver enzymes more effectively. Many types of aerobic exercise may be difficult or impossible for the elderly, especially those with musculoskeletal problems and limitations. WBV appears to improve lipid profile and reduce liver enzymes in these people when used as an auxiliary treatment method. Additionally, in several studies that have been conducted on the effects of the vibration method on the whole body, muscle mass, and bone density have also increased.26 These cases suggest that this method is very helpful, particularly for the elderly.
Study Highlights
What is current knowledge?
What is new here?
Conclusion
Our study compared the effect of aerobic exercises with and without vibration on non-alcoholic fatty liver patients. We concluded from our analysis that in non-alcoholic fatty liver patients, aerobic exercise with or without vibration reduces ALT, AST, ALP, cholesterol, and triglycerides, but does not affect LDL levels significantly. While the level of HDL in non-alcoholic fatty liver patients increases as a result of these therapies. We also revealed that vibration along with aerobic exercise reduces the levels of ALT and triglycerides in patients with NAFLD, but did not significantly influence the levels of AST, ALP, LDL, and HDL cholesterol in non-alcoholic fatty liver patients. Thus, this approach can be beneficial to patients with NAFLD who have musculoskeletal diseases or are unable to perform physical activities because of age or disability.
Limitations and suggestions of the study
The study faced limitations due to a small sample size, highlighting the requirement for a larger sample size in subsequent research. Moreover, in this study WBV was applied in conjunction with aerobic exercise, so the effects of vibration alone cannot be extracted. Thus, it is recommended that future investigations explore the effects of WBV without any additional exercise. After the intervention, biochemical tests were used to evaluate serum levels of liver enzymes and lipid profiles. Ultrasound was not used for further examination. This method is recommended for future research. The present study also faced limitation because did not consider data on insulin resistance, particularly HOMA-IR. This examination is also suggested in upcoming research. Furthermore, it is recommended in light of the beneficial effect of this method in old animal samples as compared to young animals, that future studies compare the effectiveness of this method between young and old individuals. Furthermore, we did not investigate the baseline physical activity and didn’t measure the weight of participants after intervention.
Acknowledgements
The authors would like to acknowledge Tabriz University of Medical Sciences.
Competing Interests
The authors declare that there is no conflict of interest.
Ethical Approval
This study was approved by the Ethics Committee of Tabriz University of Medical Sciences (IR. TBZMED.REC.1394.37) and registered on the website of the Iranian Clinical Trial Registry (identifier: IRCT201604162664N3, https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT201604162664N3. This study meticulously observed ethical considerations under the Helsinki Declaration. The protocol has been revised by the appropriate institutional review body.
References
- He Y, Su Y, Duan C, Wang S, He W, Zhang Y. Emerging role of aging in the progression of NAFLD to HCC. Ageing Res Rev 2023; 84:101833. doi: 10.1016/j.arr.2022.101833 [Crossref] [ Google Scholar]
- Pafili K, Roden M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol Metab 2021; 50:101122. doi: 10.1016/j.molmet.2020.101122 [Crossref] [ Google Scholar]
- Clayton-Chubb D, Kemp W, Majeed A, Lubel JS, Hodge A, Roberts SK. Understanding NAFLD: from case identification to interventions, outcomes, and future perspectives. Nutrients 2023; 15(3):687. doi: 10.3390/nu15030687 [Crossref] [ Google Scholar]
- Chung KW, Cho YE, Kim SJ, Hwang S. Immune-related pathogenesis and therapeutic strategies of nonalcoholic steatohepatitis. Arch Pharm Res 2022; 45(4):229-44. doi: 10.1007/s12272-022-01379-1 [Crossref] [ Google Scholar]
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001; 50(8):1844-50. doi: 10.2337/diabetes.50.8.1844 [Crossref] [ Google Scholar]
- Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol 1999; 94(4):1018-22. doi: 10.1111/j.1572-0241.1999.01006.x [Crossref] [ Google Scholar]
- Rong L, Zou J, Ran W, Qi X, Chen Y, Cui H. Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Front Endocrinol (Lausanne) 2022; 13:1087260. doi: 10.3389/fendo.2022.1087260 [Crossref] [ Google Scholar]
- Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol 2012; 56(1):255-66. doi: 10.1016/j.jhep.2011.06.010 [Crossref] [ Google Scholar]
- Suzuki A, Lindor K, St Saver J, Lymp J, Mendes F, Muto A. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol 2005; 43(6):1060-6. doi: 10.1016/j.jhep.2005.06.008 [Crossref] [ Google Scholar]
- Oh S, Oshida N, Someya N, Maruyama T, Isobe T, Okamoto Y. Whole-body vibration for patients with nonalcoholic fatty liver disease: a 6-month prospective study. Physiol Rep 2019; 7(9):e14062. doi: 10.14814/phy2.14062 [Crossref] [ Google Scholar]
- Berzigotti A, Saran U, Dufour JF. Physical activity and liver diseases. Hepatology 2016; 63(3):1026-40. doi: 10.1002/hep.28132 [Crossref] [ Google Scholar]
- Shantakumari N, Ahmed M. Whole body vibration therapy and cognitive functions: a systematic review. AIMS Neurosci 2023; 10(2):130-43. doi: 10.3934/Neuroscience.2023010 [Crossref] [ Google Scholar]
- Rosenberg N. Whole-body vibration enhances bone regeneration: for treatment of osteoporosis. In: Biophysical Osteoblast Stimulation for Bone Grafting and Regeneration: From Basic Science to Clinical Applications. Cham: Springer; 2023. p. 61-3. 10.1007/978-3-031-06920-8_7.
- de Oliveira RD, de Oliveira RG, de Oliveira LC, Santos-Filho SD, Sá-Caputo DC, Bernardo-Filho M. Effectiveness of whole-body vibration on bone mineral density in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 2023; 34(1):29-52. doi: 10.1007/s00198-022-06556-y [Crossref] [ Google Scholar]
- Coelho-Oliveira AC, Monteiro-Oliveira BB, de Oliveira RG, Reis-Silva A, Ferreira-Souza LF, Lacerda ACR. Evidence of use of whole-body vibration in individuals with metabolic syndrome: a systematic review and meta-analysis. Int J Environ Res Public Health 2023; 20(4):3765. doi: 10.3390/ijerph20043765 [Crossref] [ Google Scholar]
- Cochrane DJ. The Effect of Vibration Exercise on Aspects of Muscle Physiology and Muscular Performance: A Thesis Submitted in Partial Fulfilment of the Requirements for the Degree of Doctor of Philosophy, Massey University, Palmerston North, New Zealand [dissertation]. Massey University; 2010.
- Bonanni R, Cariati I, Romagnoli C, D’Arcangelo G, Annino G, Tancredi V. Whole body vibration: a valid alternative strategy to exercise?. J Funct Morphol Kinesiol 2022; 7(4):99. doi: 10.3390/jfmk7040099 [Crossref] [ Google Scholar]
- de Oliveira RG, Coutinho H, Martins MN, Bernardo-Filho M, da Cunha de Sá-Caputo D, de Oliveira LC. Impacts of whole-body vibration on muscle strength, power, and endurance in older adults: a systematic review and meta-analysis. J Clin Med 2023; 12(13):4467. doi: 10.3390/jcm12134467 [Crossref] [ Google Scholar]
- Bogaerts AC, Delecluse C, Claessens AL, Troosters T, Boonen S, Verschueren SM. Effects of whole-body vibration training on cardiorespiratory fitness and muscle strength in older individuals (a 1-year randomised controlled trial). Age Ageing 2009; 38(4):448-54. doi: 10.1093/ageing/afp067 [Crossref] [ Google Scholar]
- Khaoshbaten M, Gholami N, Sokhtehzari S, Monazami AH, Rostami Nejad M. The effect of an aerobic exercise on serum level of liver enzymes and liver echogenicity in patients with non-alcoholic fatty liver disease. Gastroenterol Hepatol Bed Bench 2013; 6(Suppl 1):S112-6. [ Google Scholar]
- Pitisuttithum P, Treeprasertsuk S. Nonalcoholic fatty liver disease (NAFLD) among older adults. Portal Hypertens Cirrhosis 2022; 1(3):184-91. doi: 10.1002/poh2.31 [Crossref] [ Google Scholar]
- Evans WJ. Exercise strategies should be designed to increase muscle power. J Gerontol A Biol Sci Med Sci 2000; 55(6):M309-10. doi: 10.1093/gerona/55.6.m309 [Crossref] [ Google Scholar]
- Reijne AC, Ciapaite J, van Dijk TH, Havinga R, van der Zee EA, Groen AK. Whole-body vibration partially reverses aging-induced increases in visceral adiposity and hepatic lipid storage in mice. PLoS One 2016; 11(2):e0149419. doi: 10.1371/journal.pone.0149419 [Crossref] [ Google Scholar]
- Haus JM, Solomon TP, Kelly KR, Fealy CE, Kullman EL, Scelsi AR. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2013; 98(7):E1181-8. doi: 10.1210/jc.2013-1229 [Crossref] [ Google Scholar]
- Bae JC, Suh S, Park SE, Rhee EJ, Park CY, Oh KW. Regular exercise is associated with a reduction in the risk of NAFLD and decreased liver enzymes in individuals with NAFLD independent of obesity in Korean adults. PLoS One 2012; 7(10):e46819. doi: 10.1371/journal.pone.0046819 [Crossref] [ Google Scholar]
- Bemben D, Stark C, Taiar R, Bernardo-Filho M. Relevance of whole-body vibration exercises on muscle strength/power and bone of elderly individuals. Dose Response 2018; 16(4):1559325818813066. doi: 10.1177/1559325818813066 [Crossref] [ Google Scholar]