J Res Clin Med. 12:35.
doi: 10.34172/jrcm.33448
Original Article
Discrepancy between IGF-1 and GH suppression test in follow-up of the treated acromegaly patients
Mahshid Naser Langroodi Data curation, Investigation, Writing – original draft, 1 
Akbar Aliasgharzadeh Supervision, 2 
Mostafa Najafipour Methodology, Writing – original draft, 3, 4 
Neda Lotfi Yagin Formal analysis, Methodology, Writing – original draft, Writing – review & editing, 2 
Farzad Najafipour Conceptualization, Data curation, Project administration, Supervision, 2, * 
Author information:
1Department of Internal Medicine, Guilan University of Medical Sciences, Rasht, Iran
2Endocrine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Young Researchers and Elite Club, Ardabil Branch, Islamic Azad University, Ardabil, Iran
4Faculty of Medicine, Azad Ardabil University of Medical Sciences, Ardabil, Iran
Abstract
Introduction:
Acromegaly, characterized by overproduction of growth hormone (GH) and insulin-like growth factor 1 (IGF1), increases the risk of mortality and morbidities. Monitoring is a crucial aspect of managing and treating of acromegaly patients. In various studies, patients with active acromegaly have shown discordance between GH and IGF-1 results after surgery. In this study, the discrepancy between these two tests in monitoring acromegaly patients was evaluated.
Methods:
The levels of IGF-1 and GH after OGTT in 49 acromegalic patients who underwent surgery at least 3 months earlier and had been referred to clinic for follow-up were analyzed. Clinical and metabolic parameters, GH nadir, and IGF-1 values were compared between groups.
Results:
Fifty-one percent of patients had normal IGF-1 based on their age and sex, and 57.1% of patients had GHn<1 µg/L. Based on IGF-1 and GHn results, 23 patients had discordant results. The most common pattern of discordance was high IGF-1 and normal GH nadir that was seen in 13 patients. A pattern of high GH and normal IGF-1 was seen in 10 patients. There was a significant difference between the corrected IGF-1 values of the high IGF-1 group and those of active disease group (P=0.001). There was no significant difference in GHn between high GHn group and active disease group (P=0.8).
Conclusion:
There is a significant inconsistency between the results of IGF-1 and GHn in monitoring of acromegaly patients. Also, when GH-0 is<1 µg/L the use of GHn does not help and there is a need for more frequent monitoring for early diagnosis of the disease recurrence.
Keywords: Acromegaly, Glucose tolerance test, Growth hormone, Insulin-like growth factor 1
Copyright and License Information
© 2024 The Authors.
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
Authors did not receive any funding to carry out this research.
Introduction
Acromegaly is mostly caused by persistent overproduction of growth hormone (GH) and insulin-like growth factor 1 (IGF-1).1,2 Systemic problems typically linked with this hormonal condition include visceromegaly, arthralgia, and soft tissue changes. The associated medical conditions also include hypertension, type 2 diabetes, sleep apnea, and carpal tunnel syndrome.3,4 Previous studies have reported that long-term exposure to GH/IGF-1 hypersecretion can increase the risk of morbidity and mortality, exacerbate psychosocial profile, and decrease quality of life.5,6
The common treatments for acromegaly include medical therapy, surgery, and radiotherapy. According to Endocrine Society Clinical Practice Guideline biochemical control in acromegaly patients is defined as GH levels to < 1.0 µg/L and normal levels of IGF1.7 According to endocrine society guideline, random measurement of IGF-1 and GH is suggested after 12 weeks, and in case of GH > 1 µg/L, GH measurement is suggested after oral glucose tolerance test (OGTT). According to the guidelines of the American Association of Clinical Endocrinologists (ACCE), biochemical remission is defined by normal IGF-1 based on age, gender, and a decrease in GH after OGTT to less than 1 µg/L after 3 to 6 months. GH is suggested to be measured at the same time after OGTT.8 A normal IGF-1 level based on age and gender and an undetectable GH level are sufficient to indicate surgical remission. However, if GH is detectable (e.g., > 1 µg/L), GH measurement after OGTT may provide important information. So measuring GH after oral glucose at the same time as measuring IGF-1 can be more efficient.9
In various studies, up to 35% of patients with active acromegaly have discordant GH and IGF-1 test results after surgery. The ACCE and Endocrine guidelines do not provide specific recommendations regarding the management of patients with discordant GH and IGF1 levels after surgery. However, since the cause of most inconsistencies is not known, it seems that monitoring both GH and IGF-1 levels in these patients can predict the changes in disease status in some patients.10 However, while there is a good consensus on the treatment algorithm for patients with acromegaly, the guideline is less clear on the optimal monitoring of these patients. Considering the vital role of monitoring in achieving improved results, we think that a re-examination of the current criteria is needed, and due to the lack of coordination between the these two tests in the monitoring of patients undergoing acromegaly treatment, we decided to determine the coordination between these two tests in the monitoring of acromegaly patients under medical and surgical treatment and its relationship with patients’ symptoms.The aim of this study was to investigate the discrepancy between these biomarkers in the monitoring of acromegaly patients after treatment.
Material and Methods
This cross-sectional study was conducted in acromegaly patients who had enrolled in acromegaly registry program and referred to the endocrinology clinic of Imam Reza hospital for treatment follow-up.
Inclusion criteria were all acromegaly patients who have undergone pituitary adenectomy surgery or medical treatment or complementary radiotherapy at least 3 months ago. Exclusion criteria included: use of pegvisomant, glomerular filtration rate (GFR) < 45, liver failure, active hepatitis, anorexia nervosa, malnutrition, uncontrolled hyperthyroidism, uncontrolled diabetes (Hba1c > 8.5), lactation, and pregnancy A clinical symptoms questionnaire was administered and biochemical tests of IGF1 and GH were measured after 75 g of oral glucose ingestion at 0, 60, and 120 minutes.
According to the guideline, IGF-1 ng/mL levels based on the age and sex of the patients and GH < 1 µg/L after OGTT were considered as the normal range. To standardize the IGF-1 test, the corrected IGF-1 was also used (the IGF-1 levels of each person divided by the upper limit of the normal in that age and sex range). The GH concentration was measured three times: at 0 minutes (baseline), 60 minutes, and 120 minutes, which is equivalent to GH0 or random in the morning, and 60 and 120 minutes after consuming 75 g of glucose (OGTT), and the lowest GH value (µg/L) was considered as GH nadir (GHn). GHn value < 1 µg/L was considered normal and values ≥ 1 µg/L were considered as abnormal or high. GH concentration was measured with a liaison device using chemiluminescence immunoassay (CLIA) technology. The analytical sensitivity of the GH test is 0.095 µg/L to 0.100 µg/L. IGF-1 levels were measured by a liaison device using Sandwich CLIA which is a kind of detection method combined double antibody sandwich method with chemiluminescence detection method. The functional sensitivity of the IGF-1 test is 10 ng/mL and the analytical sensitivity of the test is 3 ng/mL.
Statistical analysis
The normality of the variables was examined by using Kolmogorov-Smirnov test. Quantitative variables with normal distribution were presented as mean ± standard deviation (SD) and quantitative variables with non-normal distribution were shown as median, first and third quartiles and minimum and maximum values were used for qualitative variables. One-way analysis of variance (ANOVA) was used for between-group comparisons for quantitative variables with normal distribution and, if necessary, Scheffe’s post hoc test were used. Kruskal-Wallis test was used to check the difference between quantitative variables with non-normal distribution between the studied groups. Fisher’s exact and chi-square tests were used to examine the difference in qualitative variables between the studied groups. Logistic regression analysis was used to investigate the relationship and impact of each variable. Spearman test was used to investigate the association between two quantitative variables with non-normal distribution. To minimize the risk of error, a P value < 0.012 was regarded significant in the follow-up tests. P value < 0.05 was considered significant in the rest of the test. Data were analyzed using IBM SPSS Statistics (version 26; IBM Corp, Armonk, NY).
Results
This cross-sectional study was conducted on 49 post-surgical patients with acromegaly. According to the results of IGF-1 and GHn test, patients were categorized into four groups including high GH, high IGF-1, active disease, and controlled disease groups. In the active disease group, both GHn ≥ 1 µg/L and IGF-1(ng/mL) were higher than the normal range for age and sex, and in the high IGF-1 group only IGF-1(ng/mL) was above the normal range but GHn was less than 1 µg/L. The high GH group, had normal IGF-1 (ng/mL) but their GHn was greater than 1 µg/L, and in controlled disease group IGF-1 was normal and GHn was less than 1 µg/L.
The general characteristics of the study patients were presented in Table 1. The result of chi-square test was revealed that 20.4% patients were in High GH group (GHn above 1 µg/L and normal IGF-1) and 26.5% of them were in high IGF-1 group (normal GHn and IGF-1 above the normal range for their age and sex). There was significant difference in the case of age, cIGF-1 and GH concentrations among groups (P ≤ 0.05).
Table 1.
General characteristics of the study patients
|
Variables
|
High GHa
|
High IGF-1b
|
Active diseasec
|
Controlled diseased
|
P
value
|
| N (%) |
10 (20.4) |
13 (26.5) |
11 (22.4) |
15 (30.6) |
|
| Age (Mean ± SD) |
48.8 ± 9.90 |
40.7 ± 10.42 |
47.09 ± 11.10 |
53.53 ± 11.82 |
0.02 |
| Gender n (%) |
|
|
|
|
|
| Male |
1 (10) |
8 (61.5) |
7 (63.6) |
2 (13.3) |
0.09 |
| Female |
9 (90) |
5 (38.5) |
4 (36.4) |
13 (86.7) |
|
| BMI (kg/m2) |
29.43 ± 3.95 |
29.59 ± 4.06 |
29.52 ± 6.36 |
29.40 ± 5.1 |
0.99 |
| Tumor size (mm) |
16.3 ± 8.70 |
15.9 ± 7.9 |
13.2 ± 7.1 |
13.4 ± 6.53 |
0.68 |
| FBS (mg/dL) |
100.8 ± 17.25 |
103.92 ± 21.91 |
121.27 ± 58.33 |
105.07 ± 28.63 |
0.52 |
| cIGF-1 (ng/mL) |
0.64 ± 0.23 |
1.4 ± 0.42 |
2.32 ± 0.85 |
0.57 ± 0.26 |
< 0.001 |
| GH (µg/L) |
3.5 ± 2.7 |
0.5 ± 0.27 |
2.84 ± 1.8 |
0.29 ± 0.27 |
< 0.001 |
Abbreviations: GH: growth hormone, IGF-1: insulin-like growth factor-1, BMI: body mass index, FBS: fasting blood sugar.
aHigh GH group, had normal IGF-1 but their GHn was greater than 1 µg/L.
bHigh IGF-1 group only IGF-1 was above the normal range but GHn was less than 1 µg/L.
cActive disease group, both GHn ≥ 1 µg/L and IGF-1were higher than the normal range for age and sex.
dControlled disease group had IGF-1normal and GHn was less than 1 µg/L.
The quantitative concentration of corrected IGF-1 and GHn were shown in Figure 1. The IGF-1 levels in the controlled disease and in the high GH groups were significantly lower than the other two groups. Also, GHn levels in the controlled disease and in the high IGF-1 groups were lower than the other two groups. There was a significant difference between GHn concentration in the studied groups (P < 0.001).
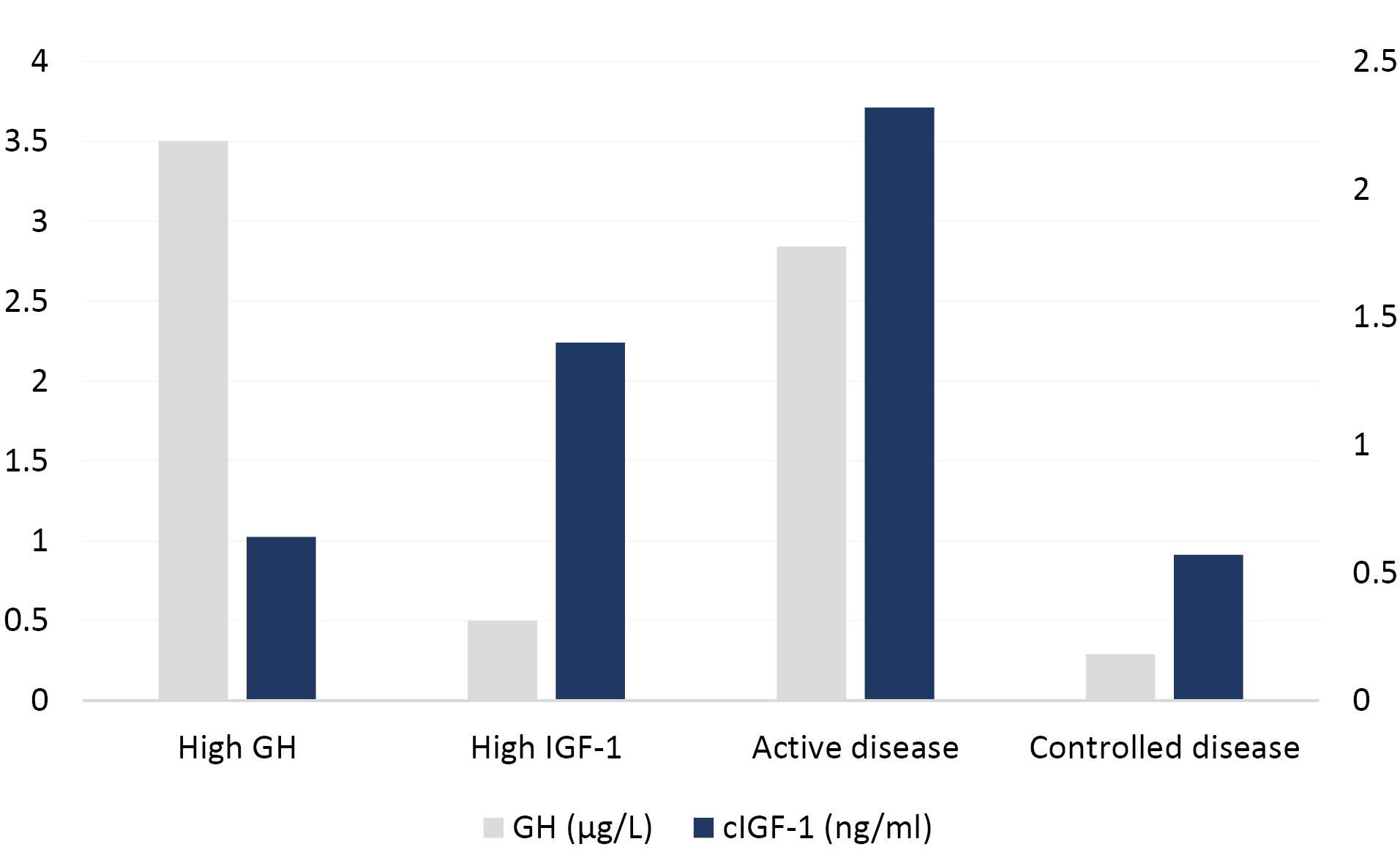
Figure 1.
The comparison the GHn and c- IGF-1 levels among groups
.
The comparison the GHn and c- IGF-1 levels among groups
There was no significant difference between the GHn value of the active disease group and the high GH group (P = 0.805), but the difference between the GHn value of the active disease group and that of the high IGF-1 and controlled disease groups were statistically significant (P = 0.002, P = 0.006). There was a significant difference between the GHn value of the high GH group and the High IGF-1 and Controlled disease groups (both P < 0.001). On the other hand, this difference between the GHn value of the controlled disease group and the High group IGF-1 was not significant (P = 0.98). (Both were less than 1 µg/L) (Figure 1).
There was a significant difference between the value of the c IGF-1 in the controlled disease group with the c IGF-1 value in high IGF-1 and active disease groups, respectively (P < 0.001, P = 0.001). Furthermore, there is a significant difference between the corrected IGF-1 value of the high IGF-1 group and active disease, controlled disease and high GH groups, respectively (P = 0.012, P = 0.001, P = 0.001). Also, there was a significant difference between corrected IGF-1 values in the active disease group and other groups, with all three groups reporting P < 0.001 (Figure 1).
Discussion
In treated acromegaly patients, the results between GH and IGF-1 tests are mostly consistent. However, in various studies, up to 35% of patients with active acromegaly have discordant GH and IGF-1 test results after surgery. The most common discordant results are the presence of GH suppression in the presence of elevated IGF-1 levels; however, normal IGF-1 levels with abnormal GH suppression are also rarely observed. The elevated concentration of both GH and IGF-1 in active acromegaly may be discordant with each other in some cases. This condition may be observed as normal GH levels with elevated IGF-1 or as normal IGF-1 with insuppressible GH.11 In the present study, the inconsistency in the form of high IGF-1 in the presence of normal GHn was seen in 26.5% of patients, but inconsistency in the form of high GHn and normal IGF-1 was seen in 20.4% of cases.
The previous evidence has shown that the main discordance was raised IGF-1 levels despite suppressed GH.12-16 The underlying mechanisms for this discrepancy are unclear, but numerous mechanisms have been suggested.17 The low continuous secretion of GH during the day or the presence of GH molecules that are biologically active was related to suppress GH with elevated IGF-1 levels.15 In addition, the independent secretion of IGF-1 from GH, which is defined as acquired autonomy of GH receptors and peripheral IGF-1 synthesis, can be regarded as another possible mechanisms.10,16,18-20 The decrease in the biological activity of GH molecules contributed to the insuppressible GH despite normal IGF-1 levels.15
Different factors including sensitivity, specificity, and standardization for accurate assessment of GH and IGF-1 are very important.21 The immunoradiometric assay or immunochemiluminescent methods are preferred to previous polyclonal radio-immunoassay methods. These parameters will help to reduce discrepancies between GH and IGF1.
In the study conducted by Alexopoulou et al, patients were divided into 4 groups: GH < 2µg/L, > 2 µg/L, IGF-1 less and more than 2 and their clinical and metabolic parameters were compared. Patients with active disease had both GH > 2 µg/L and IGF-1 z score > 2, whereas their values were low in controlled disease group. In other two groups, one group had high GH and another group had high IGF-1, while others parameters were normal. The value of IGF-1z score had a significant relationship with the average value of the GH, although it was not very strong (P < 0.001, r = 0.55). The prevalence of inconsistency in the form of high IGF-1 was 2 times greater than high GH,16 but in our study inconsistency in the form of GH ≥ 1 µg/L but IGF-1 < 2 was more common (20.4 % compared to 8.2% with GH < 1 µg/L and IGF-1 2).
The lack of discrepancy between these two biomarkers is common which can lead to delay in diagnosis and pose a challenge in drug adjustment. Furthermore, the concentration of these biomarkers is not always related to clinical symptoms, as well as other problems such as differences in laboratory assessment of GH and IGF-1 making it difficult to create a comprehensive guideline for monitoring these patients. Although, GH-dependent proteins such as IGF1BP3 or acid labile subunit have been evaluated, a new biomarker with the early disease diagnosis potential or a timely change of treatment is urgently needed.22
Study Highlights
What is current knowledge?
What is new here?
-
Both GH and IGF-1 biomarkers should be measured in the follow-up of the treatment of acromegaly patients, and for follow-up IGF-1 can be ignored in the patients with normal IGF-1 and high GH at the same time. Inconsistency in the form of high IGF-1 was observed in the presence of GHn less than 1 µg/L. Therefore, performing OGTT in GH values less than 1 µg/L does not help to evaluate the patients because in many cases GHn will be less than 1 µg/L. In the conditions of inconsistency in the form of high IGF-1, the value of this short-term biomarker is higher than the normal upper limit, and paying attention to clinical symptoms does not help in making treatment decisions, and patients should be monitored earlier in next visits
Conclusion
It can be concluded that both GH and IGF-1 biomarkers should be measured in the follow-up of the treatment of acromegaly patients, and for follow-up IGF-1 can be ignored in the patients with normal IGF-1 and high GH at the same time. Inconsistency in the form of high IGF-1 was observed in the presence of GHn less than 1 µg/L. Therefore, performing OGTT in GH values less than 1 µg/L does not help to evaluate the patients because in many cases GHn will be less than 1 µg/L. In the conditions of inconsistency in the form of high IGF-1, the value of this short-term biomarker is higher than the normal upper limit, and paying attention to clinical symptoms does not help in making treatment decisions, and patients should be monitored earlier in next visits.
The lack of data about the normal range for different age and sex and the types of binding proteins were the limitation in the assessment of IGF-1in these patients. More sensitive biomarker is needed in the presence of discrepancy between GH and IGF-1 results. It is also suggested that patients with inconsistency in test results should be monitored with a shorter interval in order to determine the progress of this inconsistency towards the recurrence of the disease or the stability of this condition or its elimination over time. This leads to early diagnosis of relapse or stable disease and appropriate treatment decision. It is also suggested to follow up patients with inconsistent results in another study to investigate the clinical future of this condition.
Acknowledgements
The authors thank all volunteers who participated in this study.
Competing Interests
The authors report no competing interest in this work.
Consent for Publication
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the study itself.
Ethical Approval
Ethical committee approval was received from the Ethics Committee of Tabriz University of Medical Sciences on December 14, 2020 (approval No: IR.TBZMED.REC.1399.878). Informed consent from participants was obtained. This study was carried out in accordance with the Declaration of Helsinki.
References
- Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13 Suppl 1:1-68. doi: 10.4158/ep.13.S1.1 [Crossref] [ Google Scholar]
- Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest 2009; 119(11):3189-202. doi: 10.1172/jci39375 [Crossref] [ Google Scholar]
- Edling KL, Heaney AP. An update on the treatment of acromegaly. Res Rep Endocr Disord 2013; 3:1-11. doi: 10.2147/rred.s24231 [Crossref] [ Google Scholar]
- Katznelson L. Drug insight: primary medical therapy of acromegaly. Nat Clin Pract Endocrinol Metab 2006; 2(2):109-17. doi: 10.1038/ncpendmet0096 [Crossref] [ Google Scholar]
- Anagnostis P, Efstathiadou ZA, Polyzos SA, Adamidou F, Slavakis A, Sapranidis M. Acromegaly: presentation, morbidity and treatment outcomes at a single centre. Int J Clin Pract 2011; 65(8):896-902. doi: 10.1111/j.1742-1241.2011.02682.x [Crossref] [ Google Scholar]
- Broersen LH, Zamanipoor Najafabadi AH, Pereira AM, Dekkers OM, van Furth WR, Biermasz NR. Improvement in symptoms and health-related quality of life in acromegaly patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 2021; 106(2):577-87. doi: 10.1210/clinem/dgaa868 [Crossref] [ Google Scholar]
- Silverstein JM. Need for improved monitoring in patients with acromegaly. Endocr Connect 2015; 4(4):R59-67. doi: 10.1530/ec-15-0064 [Crossref] [ Google Scholar]
- Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly--2011 update. Endocr Pract 2011; 17 Suppl 4:1-44. doi: 10.4158/ep.17.s4.1 [Crossref] [ Google Scholar]
- Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014; 99(11):3933-51. doi: 10.1210/jc.2014-2700 [Crossref] [ Google Scholar]
- Freda PU. Monitoring of acromegaly: what should be performed when GH and IGF-1 levels are discrepant?. Clin Endocrinol (Oxf) 2009; 71(2):166-70. doi: 10.1111/j.1365-2265.2009.03556.x [Crossref] [ Google Scholar]
- Brzana JA, Yedinak CG, Delashaw JB, Gultelkin HS, Cook D, Fleseriu M. Discordant growth hormone and IGF-1 levels post pituitary surgery in patients with acromegaly naïve to medical therapy and radiation: what to follow, GH or IGF-1 values?. Pituitary 2012; 15(4):562-70. doi: 10.1007/s11102-011-0369-1 [Crossref] [ Google Scholar]
- Halperin I, Casamitjana R, Flores L, Fernandez-Balsells M, Vilardell E. The role of IGF binding protein-3 as a parameter of activity in acromegalic patients. Eur J Endocrinol 1999; 141(2):145-8. doi: 10.1530/eje.0.1410145 [Crossref] [ Google Scholar]
- Parkinson C, Ryder WD, Trainer PJ. The relationship between serum GH and serum IGF-I in acromegaly is gender-specific. J Clin Endocrinol Metab 2001; 86(11):5240-4. doi: 10.1210/jcem.86.11.8006 [Crossref] [ Google Scholar]
- Espinosa-de-los-Monteros AL, Mercado M, Sosa E, Lizama O, Guinto G, Lopez-Felix B. Changing patterns of insulin-like growth factor-I and glucose-suppressed growth hormone levels after pituitary surgery in patients with acromegaly. J Neurosurg 2002; 97(2):287-92. doi: 10.3171/jns.2002.97.2.0287 [Crossref] [ Google Scholar]
- Machado EO, Taboada GF, Neto LV, van Haute FR, Corrêa LL, Balarini GA. Prevalence of discordant GH and IGF-I levels in acromegalics at diagnosis, after surgical treatment and during treatment with octreotide LAR. Growth Horm IGF Res 2008; 18(5):389-93. doi: 10.1016/j.ghir.2008.02.001 [Crossref] [ Google Scholar]
- Alexopoulou O, Bex M, Abs R, T’Sjoen G, Velkeniers B, Maiter D. Divergence between growth hormone and insulin-like growth factor-I concentrations in the follow-up of acromegaly. J Clin Endocrinol Metab 2008; 93(4):1324-30. doi: 10.1210/jc.2007-2104 [Crossref] [ Google Scholar]
- Zeinalizadeh M, Habibi Z, Fernandez-Miranda JC, Gardner PA, Hodak SP, Challinor SM. Discordance between growth hormone and insulin-like growth factor-I after pituitary surgery for acromegaly: a stepwise approach and management. Pituitary 2015; 18(1):48-59. doi: 10.1007/s11102-014-0556-y [Crossref] [ Google Scholar]
- Espinosa-de-Los-Monteros AL, Sosa E, Cheng S, Ochoa R, Sandoval C, Guinto G. Biochemical evaluation of disease activity after pituitary surgery in acromegaly: a critical analysis of patients who spontaneously change disease status. Clin Endocrinol (Oxf) 2006; 64(3):245-9. doi: 10.1111/j.1365-2265.2006.02430.x [Crossref] [ Google Scholar]
- Minniti G, Jaffrain-Rea ML, Esposito V, Santoro A, Tamburrano G, Cantore G. Evolving criteria for post-operative biochemical remission of acromegaly: can we achieve a definitive cure? An audit of surgical results on a large series and a review of the literature. Endocr Relat Cancer 2003; 10(4):611-9. doi: 10.1677/erc.0.0100611 [Crossref] [ Google Scholar]
- Jane JA Jr, Starke RM, Elzoghby MA, Reames DL, Payne SC, Thorner MO. Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab 2011; 96(9):2732-40. doi: 10.1210/jc.2011-0554 [Crossref] [ Google Scholar]
- Junnila RK, Strasburger CJ, Bidlingmaier M. Pitfalls of insulin-like growth factor-I and growth hormone assays. Endocrinol Metab Clin North Am 2015; 44(1):27-34. doi: 10.1016/j.ecl.2014.10.003 [Crossref] [ Google Scholar]
- Schweizer J, Schilbach K, Haenelt M, Giannetti AV, Bizzi MF, Soares BS. Soluble alpha klotho in acromegaly: comparison with traditional markers of disease activity. J Clin Endocrinol Metab 2021; 106(8):e2887-99. doi: 10.1210/clinem/dgab257 [Crossref] [ Google Scholar]