J Res Clin Med. 12():25.
doi: 10.34172/jrcm.33444
Review Article
Prevention and treatment approaches of post-dural puncture headache in obstetric patients: A comprehensive review
Afshin Iranpour Conceptualization, Writing – original draft, Writing – review & editing, 1 
Ramakrishna Boddapati Conceptualization, Writing – original draft, Writing – review & editing, 1
Sina Naghilou Writing – review & editing, 2
Amr Aly Abelazem Aly Conceptualization, Writing – original draft, Writing – review & editing, 1
Ata Mahmoodpoor Conceptualization, Writing – original draft, Writing – review & editing, 3, * 
Author information:
1Department of Anesthesiology, Al Zahra Hospital, Dubai, UAE
2Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
3Research Center for Evidence-Based Medicine, Iranian EBM Centre: A Joanna Briggs Institute (JBI) Center of Excellence, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Neuraxial anesthesia and analgesia are administered to many patients. The incidence of dural puncture is about 1%, with a higher occurrence in laboring women. Post dural puncture headache (PDPH) develops in 60%-80% of patients after unintentional dural puncture (UDP) which is usually positional and sometimes is accompanied by neck stiffness, photophobia, nausea, or hearing symptoms. It can also drive-up healthcare costs by increasing the length of hospital stay for chronic headache and back pain. PDPH has the potential to cause significant morbidity in the obstetric patients which prolongs the hospital stay. Epidural blood patch (EBP) is considered the gold standard for the management of PDPH. Proper scheduling of analgesics and supporting the patient psychologically as she cares for her newborn is needed. If the intensity of headache is severe, additional agents may be considered. In this review, the different methods and therapeutic approaches for prevention and treatment of PDPH are comprehensively discussed.
Keywords: Blood patch, Epidural, Postdural puncture headache, Primary prevention
Copyright and License Information
© 2024 The Authors.
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
No funds have been received in any forms.
Introduction
The International Headache Society (IHS) now defines Post dural puncture headache (PDPH) as, “Headache occurring within 5 days of a lumbar puncture, caused by cerebrospinal fluid (CSF) leakage through the dural puncture. It is usually accompanied by neck stiffness and/or subjective hearing symptoms. It remits spontaneously within 2 weeks, or after sealing of the leak with an autologous epidural lumbar patch.” In obstetric patients, PDPH is one of the risk factors contributing to significant morbidity. If the headache is significant or severe, the mother may not be in a position to take care of her newborn. Prolongation of hospitalization will increase the cost of maternity healthcare for both the mother and the child.1 PDPH is a common complication of diagnostic lumbar puncture and it can also occur following spinal anesthesia, or more commonly, inadvertent dural puncture during attempted epidural catheter placement. The headache is most often related with position (worse when upright, relieved on lying flat) and is often accompanied by neck stiffness and/or pain, tinnitus, subjective hearing symptoms, photophobia and nausea.2 This article attempts to summarize the current state of knowledge regarding pathophysiology, diagnosis and different therapeutic approaches based on the literature review.
Pathophysiology
Although the pathophysiology of PDPH though unclear, sagging of cerebral structures following dural puncture induced CSF leakage has been radiologically demonstrated.3 This mechanism is consistent with MRI in several reported cases of PDPH. Intracranial hypotension related to CSF leakage may cause sagging of intracranial structures and stretch of sensory intracranial nerves, causing pain and cranial nerve palsies. If CSF leaks at a rate greater than the rate of CSF production, low CSF pressure can result, accentuated at the level of the brain in the upright position.3 Concurrent intracranial hypotension causes traction of intracranial structures and induces a compensatory adenosine-mediated dilation of the meningeal vasculature, as reported in some cases.4 However, not all patients with PDPH have low CSF pressure; and not all patients with significant CSF leak develop a headache. CSF hypotension results in compensatory meningeal venodilation and blood volume expansion, with headache caused by acute venous distension. Altered craniospinal elasticity after lumbar puncture results in increased caudal compliance relative to intracranial compliance and acute intracranial venodilation in the upright position.
Methods
The PubMed database was searched with the following search terms: (“Post-Dural Puncture Headache/prevention and control”[Mesh]) AND “Obstetric Surgical Procedures”[Mesh]. Additionally, 4 articles were identified using the hand‐searching method. The articles were included in this review if their titles or abstracts were available in English and presented prevention and treatment approaches for post-dural puncture headache in obstetric patients. The most current literatures are tried to include. Studies that were not related to gynecology and obstetrics were excluded (Figure 1). Tables 1 and 2 present the studies regarding the post-dural puncture headache in obstetric patients.
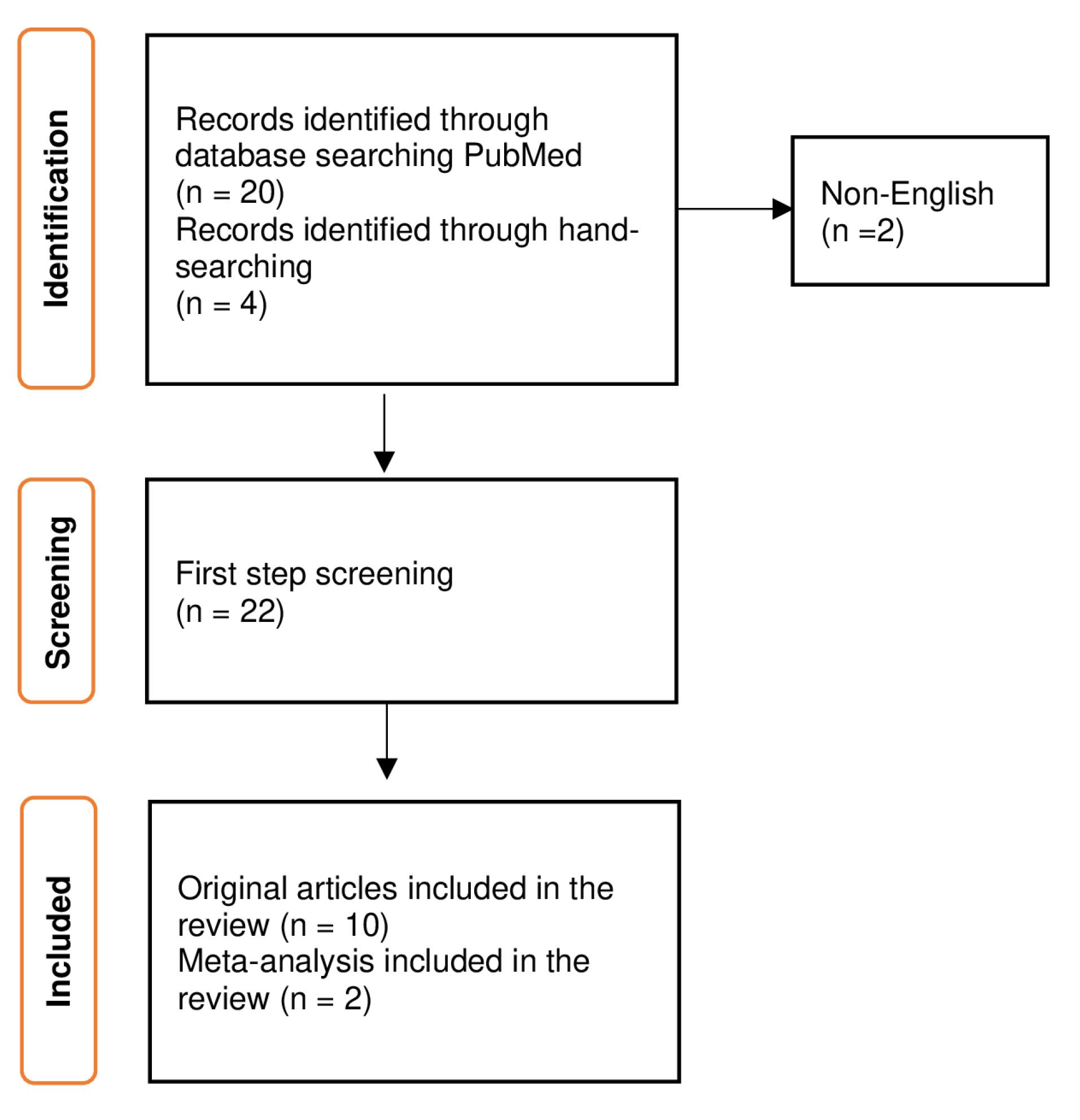
Figure 1.
Selection process of the articles
.
Selection process of the articles
Table 1.
A summary of the clinical trials regarding the PDPH in gynecological patients
|
Study
|
Year
|
Country
|
Study type
|
Type blinding
|
Sample size
|
Group A
|
Group B
|
Initial dose of group A
|
Initial dose of group B
|
Population
|
Result
|
| Fattahi5 |
2013 |
Iran |
Randomized clinical trial |
Double-blinded |
212 |
106 |
106 |
Ondansetron 0.15mg/kg + 5ml normal saline |
5ml normal saline |
Between 20- and 40-year-old patients who candidate for elective cesarean section under spinal anesthesia |
PDPH in the intervention group was considerably less than in the control group |
| Hakim6 |
2006-2009 |
Egypt |
Randomized clinical trial |
Double-blinded |
95 |
47 |
48 |
Cosyntropin 1mg |
equal volume of normal saline |
Patients in labor who suffered an accidental Dural puncture |
cosyntropin reduced significantly the incidence of PDPH |
| Al‐Metwalli7 |
2003-2007 |
Saudi Arabfia |
prospective, randomized, trial |
double-blinded |
50 |
25 |
25 |
3mg morphine in 10ml saline |
10ml saline |
Obstetric patients who had an inadvertent Dural puncture |
epidural morphine is efficient method for prevention of post Dural puncture headache |
| Riveros Perez et al8 |
2016-2018 |
Georgia |
Retrospective observational study |
|
32 |
14 |
18 |
1 mg
of IV cosyntropin,
3 mg of epidural morphine, and 60 cc of epidural normal saline |
other methods |
Patients with an accidental Dural puncture after labor epidural anesthesia |
The triple prophylactic (epidural saline, IV cosyntropin, and epidural morphine) after
accidental dural puncture reduced the incidence of PDPH. |
| Charsley and Abram9 |
1997-1999 |
USA |
prospective, randomized, trial |
Not blinded |
48 |
22 |
26 |
10 mL of normal saline injected into the subarachnoid space |
no saline injection |
Obstetrical patients who experienced accidental dural puncture |
Administering 10 mL of normal saline through an intrathecal injection immediately after a wet tap can decrease the occurrence of post-dural puncture headache and the requirement for epidural blood patch. |
| Okpala et al10 |
2020 |
Nigeria |
Randomized clinical trial |
Double- blinded |
192 |
96 |
96 |
2mls (8 mg) of dexamethasone |
2mls of normal saline |
All parturients who underwent cesarean section under
spinal anesthesia |
Giving prophylactic dexamethasone can lower the occurrence and intensity of PDPH, both on the first and fourth day after spinal anesthesia for cesarean delivery. |
| Yang et al11 |
2018 |
China |
Randomized clinical trial |
Double- blinded |
120 |
60 |
60 |
250 mg aminophylline intravenously |
equal volume of normal saline |
Patients undergoing elective caesarean
section |
One double-blind clinical trial study showed that injection of 250 mg aminophylline intravenously effectively prevented the occurrence of PDPH after caesarean section. |
| Stein et al12 |
1997-2005 |
USA |
Randomized control trial |
Single-blinded |
116 |
60 |
56 |
prophylactic epidural blood patch |
conservative treatment with a therapeutic epidural blood patch if required |
Patients who had undergone accidental dural puncture during epidural block for labour analgesia or caesarean delivery |
the study show that prophylactic epidural blood patch is a useful approach to reduce the development of post-dural puncture headache in obstetric patients. |
| Yousefshahi et al13 |
2012 |
Iran |
randomized clinical trial |
double-blinded |
360 |
182 |
178 |
8 mg
(2 ml) of dexamethasone was intravenously |
2ml normal saline |
patients who were
scheduled to undergo cesarean section under spinal anesthesia |
Prophylactic dexamethasone increases the severity and incidence of PDPH. |
| Faridi Tazeh-kand et al14 |
2009 |
Iran |
randomized clinical trial |
double-blinded |
100 |
50 |
50 |
Intrathecal normal saline 5ml before intrathecal injection of 2.5ml (12.5mg) hyperbaric bupivacaine 0.5 % |
2.5 ml (12.5 mg) hyperbaric bupivacaine 0.5 % as a control |
healthy women of age between 18 and 35 years who underwent elective term cesarean delivery under spinal anesthesia |
Preventive 5ml of normal saline before intrathecal administration of hyperbaric bupivacaine is a practical method for reducing PDPH in patients undergoing cesarean section. |
PDPH: Post dural puncture headache.
Table 2.
A summary of the systematic reviews regarding the PDPH in gynecological patients
|
Study
|
Study design
|
Year
|
Number of included studies
|
Population
|
Type of treatment or prevention
|
Result
|
| Lee et al15 |
Meta-analysis |
2018 |
20 |
4936 patients undergoing Cesarean section with spinal anesthesia |
spinal needle types |
This meta-analysis shows that using the pencil-point spinal needle decreases the incidence of PDPH in patients undergoing Cesarean section without increasing any possible adverse effects. |
| Zhao et al16 |
Meta-analysis |
2023 |
22 |
4,921 pregnant women |
aminophylline, dexamethasone, gabapentin/pregabalin, hydrocortisone, magnesium, ondansetron, and propofol |
According to this meta-analysis, propofol, ondansetron, and aminophylline decrease the incidence of PDPH compared to the placebo group. |
PDPH: Post dural puncture headache.
Risk factors
Patient risk factors
Age, in most studies, extremes of age are associated with lower incidence of PDPH, with young adults (less than 40 years old) having the highest risk. In the elderly, reduced elasticity of the dura might be responsible for lesser likelihood of gaping following dural puncture. Extensive evidence supports the observation that PDPH is uncommon in patients older than 60 years of age and is most common in patients younger than 40 years of age. A systematic review indicates that female (non-pregnant) gender may be a risk factor for the development of PDPH. Non-pregnant females have approximately twice the odds of developing a PDPH compared with males.17
Pregnancy confers an additional risk for PDPH, its high incidence may be attributed to increased estrogen levels, which influence the tone of the cerebral vessels, thereby increasing the vascular distension in response to CSF hypotension. Vaginal Delivery, following documented dural punctures, as compared to parturients who never pushed for delivery, those subjected to active bearing down had higher incidence of PDPH and epidural blood patch (EBP) associated with them. There may also be a time-dependent association between the cumulative duration of bearing down in second stage labour and the incidence of PDPH.18 Recent reports suggest that cesarean performed during the first stage of labour greatly reduced the rate of PDPH in parturients who failed to reach the second stage of labour after unintentional dural puncture (UDP). The valsalva maneuver during the second stage of labor may increases the size of the dural tear after UDP with a Tuohy needle.For this reason, some experts use forceps for delivery in order to shorten the second stage of labour to reduce maternal pushing after UDP. According to few research hypotheses, obesity (BMI > 31.5) increases intra-abdominal and epidural pressures. Reduced CSF leakage following dural puncture, due to decreased pressure gradient between subarachnoid and epidural spaces in obese individuals, might be the reason for lesser likelihood of PDPH.19 History of prior headaches, there is a close relationship in the development of a postdural puncture headache and those with history of chronic headache.20
Both previous PDPH and chronic headaches may be a risk factor for PDPH; although, this has not been observed in all studies. Cigarette smokers had a lower incidence of PDPH than nonsmokers; possibly due to the alterations in coagulation properties of smoking that facilitate the occlusion of the dural puncture, or stimulation of dopamine neurotransmission by nicotine.21 In a retrospective study that assessed the influence of cigarette smoking on the risk of PDPH among patients who had continuous CSF sampling via catheter, cigarette smokers were found to have a lower incidence of PDPH compared with nonsmokers (13.7 vs 34.1%). Although the mechanism is not clear, maybe the clot-promoting properties of smoking, dopamine production, and vasoconstriction property of noradrenaline have a role to play. The authors proposed that there is occlusion of the dural puncture by clot. Stimulation in the production of dopamine, the CNS reward property of which limits the severity of PDPH and conversion of dopamine to noradrenaline, which counteracts the intracranial vasodilatation associated with PDPH.22
Procedural risk factors
Needle tip, Whitacre and Sprotte needles, which are the most commonly used pencil point (atraumatic/non-cutting) needles, have a diamond- shaped tip with the orifice is situated up to 0.5 mm from the needle tip.15,23 The Quincke needle, which is the most commonly used conventional, or cutting, spinal needle, has a sharp cutting tip with the hole at the end of the needle.
In a meta-analysis conducted by Zhang and colleagues, using spinal needles of Whitacre group showed reduced incidence of PDPH and severity of PDPH. Also the need for an EBP was more in the Quincke group compared to Whitacre group.24 Electron microscopy has shownthat Whitacre needles are actually more traumatic to the dura compared with the Quincke needles. It is suggested that perhaps the trauma from the pencil point needle results in greater inflammatory reaction that aids in early sealing of the dural tear.25 Contact with bone during needle insertion, whether cutting or spreading type, may lead to tip deformation. Damaged tips could contribute to an increased incidence of PDPH. Recent in vivo studies have demonstrated
that beveled spinal needles are more likely to deform after bony contact than comparable sized pencil-point needles.26 Experts recommend using spinal needles with a pencil point tip rather than needles with a sharp cutting tip (Table 3). Needle size is directly related to the size of the dural tear and the risk of PDPH. The influence of needle size on risk of PDPH appears to be greatest for cutting needles.In one study, the incidence of PDPH has reported to be between 0.7% and 4% when 24–27 G atraumatic needles are used for spinal anesthesia.27 The most common cause of severe headache now is UDP with an epidural needle (16 to 18-gauge) during epidural placement, the incidence of PDPH can be more than 70%28 (Table 3). Needle orientation. As the collagen fibers in the dura mater run in a longitudinal direction, orienting the needle bevel parallel to the longitudinal dural fibers significantly decreases both the incidence and severity of headache, and the need for therapeutic EBP.29 The incidence of PDPH following Quincke needles may not only be affected by the direction of the bevel during insertion but also during removal. No PDPH occurred when the bevel was inserted and removed parallel to the dural fibers.30 Tearing of the dura may occur upon needle removal if it is rotated to a perpendicular orientation after insertion. Bevel orientation is not an issue with atraumatic needles as they separate the dural fibers rather than cutting them allowing them to return to their original position with decreased CSF leakage.31 With a cutting needle, reinsertion of the stylet may not affect the rate of PDPH, however with a Sprotte (pencil point) needle, reinsertion of the stylet before removing the needle appears to lower the risk of PDPH. It is thought that a strand of arachnoid mater may be reintroduced into the CSF when the needle is withdrawn without stylet replacement, thereby prolonging the CSF leakage. The incidence of PDPH after spinal anesthesia is much lower than after diagnostic LP. When CSF is collected during diagnostic LP, along with CSF a strand of arachnoid may enter the spinal needle, which gets threaded back through dura upon needle removal, resulting in CSF leakage. Replacing the stylet up to the tip of the needle should push out or cut off the strand and thus reduce the frequency of PDPH.32
Table 3.
Frequency of PDPH with needle tips and needle size
22,31,33
|
Needle tip
|
Needle size
|
Frequency of PDPH (%)
|
| Tuohy |
16 |
70 |
| 18 |
64 |
| Quincke |
20 |
40 |
| 22 |
36 |
| 24 |
11.2 |
| 25 |
6.4 |
| 26 |
5.6 |
| 27 |
2.9 |
| Whitacre |
20 |
2-5 |
| 22 |
1.5 |
| 25 |
2.0 |
| 27 |
1.6 |
-
Ondansetron: 0.15 mg/kg.
-
IV Hydrocortisone: 200 mg stat followed by 100 mg 3 times a day for 48 hours*.
-
ACTH: 1 mg dose*.
-
Epidural Morphine: Two 3 mg doses in 10 ml saline, 24 hours apart.
-
Intrathecal Saline: 10 ml.
-
Caffeine: 300 mg - 500 mg, oral or IV once or twice daily.
-
Acetaminophen: 1 gram every 6 hours.
-
Ibuprofen: 400 mg every 6 hours.
-
ACTH: injections 1.5 mic/kg in 500 mL saline IV over 30 minutes.
-
Gabapentin: 300 mg every 8 hours for 4 days.
-
Pregabalin: 150 mg/day for 3 days, then 300 mg/day for 2 days.
-
IV Hydrocortisone: 100 mg IV every 8 hours for 2 days.
-
Methylprednisolone: One IV dose of 500 mg.
-
Aminophylline: IV 250 mg
-
Theophylline: IV 200 mg over 45 min, 2 doses.
-
20% Mannitol: 100 ml every 12 hours for 48 hours.
-
Sumatriptan: 50 mg oral every 8 hours up to 5 days.
* Prophylactic dose.
Regarding technique, placement of spinal needle in the sitting as opposed to lateral position, and more attempts with needle passes, may be associated with increased risk for PDPH in spinal anesthetics. The potential benefits of lateral decubitus position for preventing PDPH can be explained by the differences in CSF pressures. Sitting position is associated with a higher CSF pressure of 40 cm H2O as compared to 5-20 cm H2O in lateral position. The higher pressure is hypothetically associated with a larger hole and a prolonged leak at a higher pressure. Further displacement of brain matter and meninges occur earlier in sitting position, resulting in more symptoms. This downward movement does not occur in lateral position leading to lower risk of developing PDPH.34 An angled approach to the subarachnoid space, such as a paramedian approach, is associated with a lower incidence of PDPH.35 In vitro model of dural puncture, a 30 degree angle of approach to the dura with 25G Quincke needles resulted in significantly lower leak rates than with the same needles used to puncture the dura at 60 or 90 degrees. A possible explanation for this difference is that an oblique needle track through a thick membrane such as dura may tend to seal itself via a flap valve mechanism. Such a flap would tend to close when fluid pressure is applied to one side of the membrane. In addition, the holes made in the dura and arachnoid membranes may be offset enough following an oblique needle puncture to provide a second flap valve mechanism. A 30 degree needle angle can be achieved clinically using a lateral approach for lumbar puncture.36
In epidural placements, studies have failed to show a difference in PDPH risk with the use of air versus saline for the loss of resistance technique to identify the epidural space across all patient populations. The onset of headache may be sooner in obstetric patients when air is used for loss of resistance. Rotating an epidural needle once inside epidural space may increase the risk of dural puncture.
The number of lumbar puncture attempts directly relates to the size of the dural damage, making fewer attempts at dural puncture could be associated with lesser incidence of headache after lumbar puncture.23 In one study, the number of attempts required for a successful subarachnoid block is less with 25G than 27G Whitacre needle.37 Anesthesia providers with relatively lesser experience or expertise (those having done 10 epidurals or less) had an UDP rate of 2.5%, which was twice the rate of 1.3% in the Anesthesia providers with relatively more experience or expertise (those having done 90 epidurals or more).
Incidence
PDPH related to neuraxial anesthesia is most common in obstetric patients and its incidence varies widely, depending on patient and procedural risk factors. UDP occurs in less than 1 percent to as high as 6% of epidural placements and more than 70% of these patients develop PDPH.31 If UDP is not recognized (11% to 33% of UDP) at once, either by seeing CSF coming from the epidural needle or catheter, or by the onset of an unexpectedly profound block after a test dose, the patient is at risk of respiratory failure from a high block if a larger dose of local anesthetic is delivered into the catheter in the subarachnoid space.38 Inadvertent dural puncture does occur with similar frequency following spinal/epidural anesthesia (CSE) compared with lumbar epidural anesthesia. The reduced incidence of PDPH following CSE may be due to the epidural needle acting as an introducer for the spinal needle, reducing the number of attempts to puncture the dura using the spinal needle. On the other hand, 25 to 27 gauge spinal.
Needles are used for CSE, and the incidence of PDPH following their use is low (less than 2%). Also, the injection of local anesthetic into the epidural space during CSE reduces leakage of CSF through the dura secondary to increased epidural pressure and finally, epidural opioids may provide a prophylactic effect against PDPH.35
Clinical finding and diagnosis
The headache features of PDPH are variable but are typically moderate to severe in intensity and orthostatic in nature. Patients with PDPH typically present with frontal or occipital headache that is worse with sitting or standing, and relieved by lying flat.39 The International Headache Society (IHS) diagnostic criteria further describe this positional quality as worsening within 15 minutes of sitting or standing and improving within 15 minutes after lying. The patient would need at least one of the following symptoms in association with the headache: neck stiffness, tinnitus, hypoacusia, photophobia, or nausea.28
Ninety percent of headaches begin within 72 hours but may rarely occur 5–14 days after the procedure.33 PDPH is reported to resolve spontaneously in more than 50% of patients within 4 days, and in more than 70% within a week, whereas 87% of the cases resolve within 6 months.21,27,35 Associated symptoms occur in up to 70% of patients, and may include nausea, vomiting, neck stiffness, low back pain, vertigo, dizziness, diplopia, photophobia, cortical blindness, cranial nerve palsies, and even seizures. Auditory changes commonly seen and present as tinnitus and decreased hearing in the low frequency range. Diagnostic lumbar puncture (LP) should be avoided if possible, because of the risk of worsening an existing PDPH. If a diagnostic lumbar puncture is performed, it may show a low CSF opening pressure, a slightly raised CSF protein and a rise in CSF lymphocyte count. If alternative diagnoses need to be ruled out, CT/MRI (neuroimaging) may be considered. The findings of MRI of the brain may be consistent with diffuse dural enhancement and evidence of sagging, descent of the brain and brainstem, obliteration of the basilar cisterns and enlargement of the pituitary gland might be seen. These findings are analogous to those reported in patients with spontaneous intracranial hypotension.23
Differential diagnosis includes tension-type/migraine headache, preeclampsia/eclampsia, musculoskeletal headache, drugs withdrawal (i.e. caffeine), sinus headache, meningitis (viral, chemical, or bacterial), intracranial hemorrhage, cerebral infarction, intracranial tumor, pituitary apoplexy, cerebral sinus thrombosis, and non-specific headaches.28 This must be kept at the back of mind especially in the absence of a postural relationship with headache. If the patient presents with focal deficits or neurological deficits become worse, it becomes necessary to rule out life threatening causes (e.g., hemorrhage, thrombosis, vasculopathy, intracranial hemorrhage, cerebral infarction, intracranial tumor, pituitary apoplexy, cerebral sinus thrombosis, meningitis. When performing epidural loss-of-resistance technique using air, pneumocephalus (PNC) must be kept in mind if symptoms occur within an hour of the procedure. PNC tends to be small and to resolve spontaneously and hyperbaric oxygen therapy may be useful in the management of symptomatic persistent PNC.
Management
Prevention of PDPH after dural puncture
summary of the PDPH management methods is presented in Figure 2. As approximately 50% of UDP cases land up with PDPH, it is reasonable to employ prophylactic measures. It will help reduce any morbidity risk that may develop, although it is important that the prophylactic procedure itself is not associated with significant risks.1
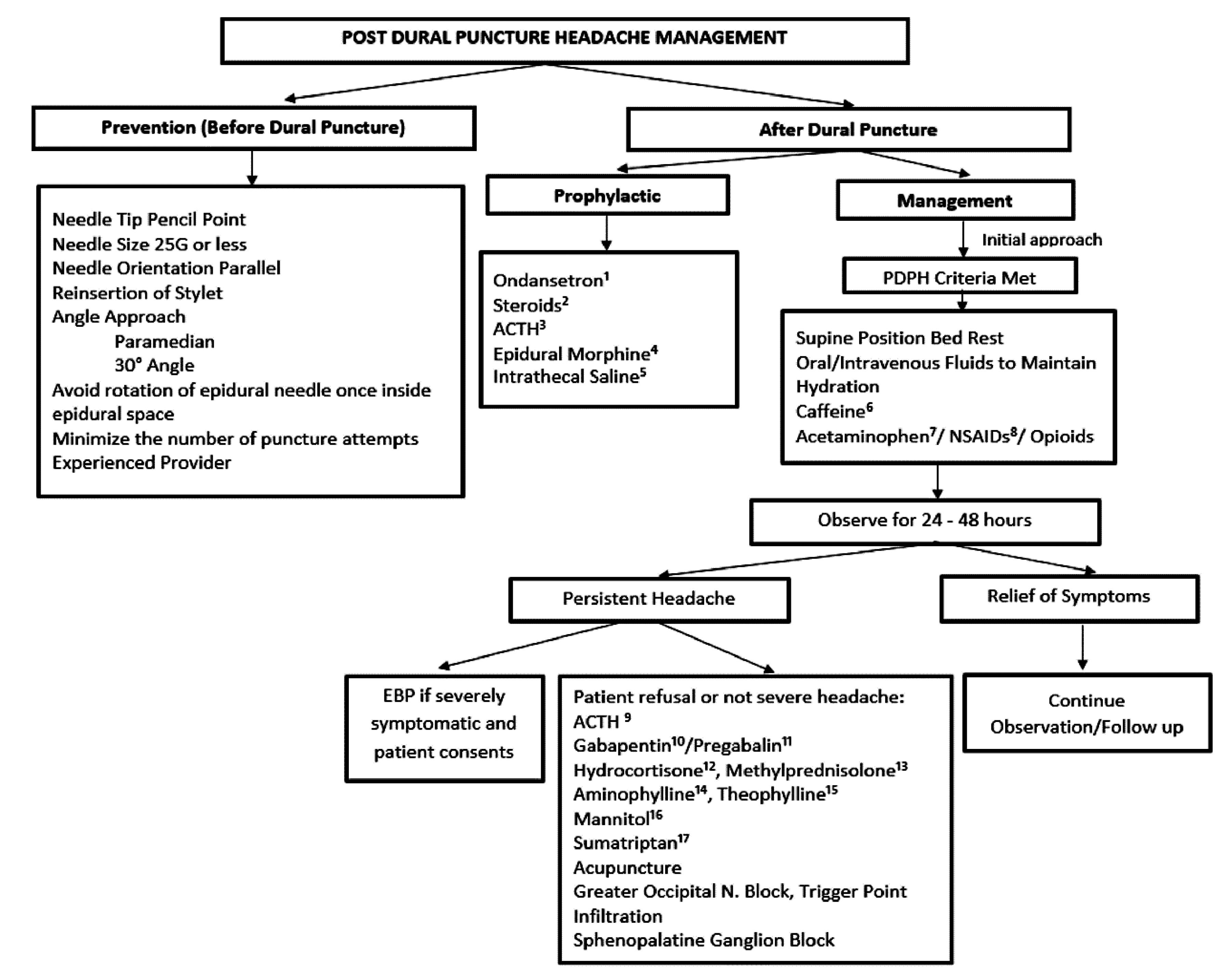
Figure 2.
Summary of PDPH management
.
Summary of PDPH management
Bed rest
Has not been shown to significantly decrease the risk of PDPH but still continues to be recommended. Hypercoagulable state in pregnant women puts them at risk of deep venous thrombosis and pulmonary embolism and immobility can further increase the risk.
Oral fluids
Evidence is found lacking in regards to greater hydration preventing PDPH, although it’s been a common practice to encourage intake of oral feeds in the women following UDP so, normal hydration should be maintained.3
Abdominal binders
Theoretically, the abdominal compression achieved by abdominal binders increases the intra-abdominal pressure that may be transmitted to the epidural space. This decreases the CSF leak and may seal the puncture site and causing an improvement in existing headaches.
Caffeine
The role of oral caffeine is negligible in obstetric and general surgical patients in preventing PDPH following dural puncture.
Ondansetron
In one double-blind randomized placebo controlled study, it has been reported that prophylactic administration of ondansetron (0.15 mg/kg) may reduce the incidence of PDPH after spinal anesthesia for cesarean.5
Steroids
Although inconclusive, there is a study that hints at possible benefits following the use of dexamethasone and hydrocortisone in prevention of PDPH. In a study, prophylactic intravenous administration of dexamethasone was administered and the severity was found to be reduced, however its incidence was not significantly affected. As well, intravenous hydrocortisone 200 mg stat, followed by 100 mg three times daily for 48 hours was shown to be effective in reducing the severity of PDPH among a sample of obstetric patients.22 In another study, giving prophylactic 8mg of dexamethasone intravenously can lower the occurrence and intensity of PDPH, both on the first and fourth day after spinal anesthesia for cesarean delivery.10 Although, a randomized clinical trial demonstrates that prophylactic dexamethasone increases the severity and incidence of PDPH.13
Adrenocorticotropic hormone (ACTH) and analogues
The mechanism of action of cosyntropin (a synthetic ACTH analogue) is unknown but may be related to an aldosterone-stimulating effect on volume expansion. Due to the resultant increased circulating volume, it causes dural edema and increases CSF production (by enhanced sodium ion transport), both of which promote the closure of the dural puncture. There may also be an associated increased central beta-endorphin production which decreases pain perception.7,15,22 Significant reduction in the incidence of PDPH and the need for EBP was seen following administration of a single intravenous dose (1 mg) of cosyntropin following UDP. Cosyntropin administration was also associated with significant prolongation of the time from UDP to occurrence of PDPH, although it did not influence either the duration or the severity of the headache.6
Epidural morphine
Results of a prospective, randomized, double-blinded, placebo-controlled trial in which epidural morphine was administered as two 3 mg doses in 10 ml saline 24 hours apart, showed the incidence of PDPH of 12% in the with-morphine group as compared to 48% in normal saline without-morphine group. Epidural morphine appears to be a simple and effective technique for prevention of PDPH after UDP in high risk obstetric patients.7 In order to use epidural morphine for the prevention of headache, it requires that the patient stays in the hospital for 46 hours after delivery.39
Epidural saline
The injection of saline solution into the epidural space is believed to temporarily equilibrate the pressure, which should minimize the leakage of CSF through the dural tear long enough for a fibrin seal to block the aperture. There have been many regimens proposed for this therapy. One is to inject 40 mL of saline into the epidural space, and then an infusion of 40 mL/h over the next 12-24 hours. Another one is two injections of 60 mL saline, first one administered immediately after delivery and the second one administered the following morning. There is currently insufficient evidence to recommend the use of prophylactic epidural saline injection in obstetric PDPH.1,3,31 Although in the randomized clinical trial performed in Iran, preventive 5ml of normal saline before intrathecal administration of hyperbaric bupivacaine is a practical method for reducing PDPH in patients undergoing cesarean section.14
Multimodal approach (Triple prophylaxis)
The multimodal approach protocol (triple prophylaxis) consists of the immediate transcatheter administration of 60 mL of epidural normal saline when the diagnosis of a wet tap is made, and immediately after delivery 1 mg of intravenous cosyntropin, and 3 mg of epidural morphine before epidural catheter removal. It has the potential to reduce both the incidence of PDPH and the need for blood patch treatment. The effect may be explained by the synergistic effect of different mechanisms (analgesic effect of morphine, the pressure-equalizing effect of saline, and the increased production of CSF and endorphin release induced by cosyntropin) of action targeting multiple levels in the complex pathophysiology of PDPH.8
Prophylactic epidural blood patch
Prophylactic blood patch may be difficult to justify when the headache is not present; also, the lack of evidence for its effectiveness may have contributed to its decline.40 The prophylactic blood patch is usually performed at least 5 h after the last dose of epidural anesthetic by injecting 15-20 ml of blood through the epidural catheter before pulling it.28 A review of the literature of prophylactic EBP in obstetric patients found that it does not appear to decrease the incidence of PDPH, but may decrease the intensity and/or duration of symptoms (Figure 3).41
Intrathecal catheter placement
Routine placement of an intrathecal catheter is not advised, however placing intrathecal catheters selectively (e.g., after a difficult epidural procedure) is reasonable. Spinal catheters may not prevent PDPH, but they reduce the need for multiple epidural attempts and the risk of subsequent, additional UDP. Re-sited epidural catheters after UDP appear to perform better in comparison to intrathecal catheters as regards labour analgesia, with higher failure rate seen in intrathecal catheter patients after UDP.
A meta-analysis looking into intrathecal catheter placement after UDP showed that it significantly reduces the need for an EBP without reducing the incidence of PDPH.42
Intrathecal saline
The immediate injection of 10 mL intrathecal normal saline after an UDP significantly reduced the incidence of PDPH and the need for EBP. When an intrathecal catheter had been placed following an UDP, injection of 10 mL of normal saline before its removal effectively prevented PDPH.9
Treatment
Pharmacological
Simple analgesics
Simple oral analgesic medication such as acetaminophen (1 g every 6 hours), NSAIDs (e.g., ibuprofen 400 mg every 6 hours) and weak opioids including codeine or tramadol should be offered to women with postnatal headache.3
Caffeine
The proposed mechanism of action of caffeine in PDPH is cerebral vasoconstriction and increased CSF production.3 Intake of oral caffeine in daily coffee drinkers is encouraged, so as to avoid headache and withdrawal symptoms, by some experts. Despite its widespread use, there is limited evidence to support the use of caffeine in the treatment of obstetric PDPH. If used, treatment with caffeine should not exceed 24 hours, oral therapy is preferred, and doses should not exceed 300 mg (or 500 mg intravenous), with a maximum of 900 mg in 24 hours. A lower maximum dose of 200mg in 24 hours should be considered for women who are breastfeeding, particularly those with low birth weight or premature infants. Patients on caffeine therapy must monitor the amount of caffeine in their cup of beverage and daily recommended doses of caffeine should be adhered to.3,43
Opioid
In situations where simple oral analgesia is not effective, stronger opioid medication such as morphine or oxycodone can often be given to women with PDPH. Given the risk of side effects, long-term therapy ( > 72 hours) is not recommended.3
ACTH and analogues
Exact mechanism yet to be ascertained, few hypotheses have been suggested to explain how ACTH or their synthetic analogues such as tetracosactrin (Synacthen) and cosyntropin help in relieving PDPH. First, the release of aldosterone secondary to ACTH results in retention of salt and water leading to edema formation especially in the torn dural edges thus minimizing CSF leakage. Second, production of CSF is increased secondary to the active Sodium ion transport system, favored by ACTH. Third, likely as a result of pain perception modulation due to brain β endorphin levels going up. It has been shown in vitro that ACTH-derived fragments may mimic the effects of morphine, thereby relieving pain. Results of a study advise two injections of ACTH 12h apart (1.5 μg/kg in 500 mL saline intravenous over half an hour) if no improvement is noted in the first 24h conservative management with an efficacy of 90%.44
Gabapentinoids
Both medications have been shown to be effective in reducing the severity of pain associated with PDPH in their individual studies. Gabapentin also appeared to be useful in the majority of cases when EBP had been refused or was contraindicated. The typical dose of gabapentin used in these studies is 300 mg every 8 hours for 4 days and pregabalin is 150 mg/day for 3 days then 300 mg/day for a further 2 days. The associated risks of potential neurotoxicity in the newborn in breast-feeding mothers’ limits its readiness for use in all patients with PDPH.45-47
Steroids
The successful use of hydrocortisone and methylprednisolone, statistically, has been reported significant improvement in the severity of headache. Hydrocortisone 100 mg IV 8th hourly for 48 hours showed a reduction in VAS scores after 6 hours.48 Methylprednisolone (500 mg one dose) along with conventional therapy showed reduction in VAS scores after 6 hours compared to only conventional therapy group.49
Aminophylline and theophylline
Intravenous (IV) aminophylline (250 mg) is a safe and effective early treatment for PDPH according to a randomized study, if the treatment started within 3 hours of symptom onset. Analysis indicated that treatment with aminophylline resulted in significantly lowered pain in comparison with placebo within 30 minutes, and that this improvement remained at the 2-day follow-up.11,16,50 Oral theophylline has been used with some positive effect for PDPH, but has psychogenic side effects (11). In one study by Utku Yıldırım et al the mean VAS scores of the patients after theophylline infusion decreased significantly compared to baseline.51
Triptans
Sumatriptan is a serotonin receptor agonist that has been used (50 mg three times daily (TDS) orally for up to 5 days) when EBP was contraindicated or declined (with 20% success rate). It is not licensed for administration if the mother is breastfeeding.38
Mannitol
The use of mannitol 20%, 100 mL infusion over ½ hours followed by 100 mL every 12 hours settled PDPH after the first dose for 6–8 hours and there was no need for mannitol infusion after 48 hours.48
Other medications
Stool softeners and a soft diet will help decrease valsalva straining and leakage of CSF. If nausea or vomiting is prominent, 10 mg metoclopramide orally every 8 hours is allowed.6
Intravenous fluids
Intravenous fluid therapy may be used when a patient is unable to take adequate oral fluids, especially when dehydrated.3
Invasive procedures
Patients who do not respond to conservative treatment within 48 hours are subjected to
Invasive treatments.25
Epidural blood patch
EBP remains as the gold standard for the treatment and prevention of PDPH.2,12 EBP reduced the duration and intensity of post-dural puncture headache.52 For cases of persistent PDPH resistant to conventional EBPs, considerations should be given to CT guided EBP.53 Fluoroscopically guided EBP can be used to treat persistent PDPH with relatively small volume of blood for epidural injection.54,55 Optimum time for an EBP to ensure greater efficacy is usually to perform the procedure after 48 hours of dural puncture and performing it within 48 hours of dural puncture is associated with greater requirement for a repeat EBP. In severe obstetric PDPH, an EBP within 48 hours of dural puncture may be considered for symptom control, although it may need to be repeated.56
Two theories have been proposed to try and explain the mechanism by which EBP helps in the management of PDPH. Firstly, autologous blood injected in the epidural space forms a clot that gets adhered to the dura mater and results in direct patching of the hole.57 The second hypothesis suggests that there is reduction in traction of the pain sensitive brain structures due to increase in CSF pressure secondary to the volume of blood injected.58
Procedure
A thorough medical history and physical examination should be followed by observing for any signs of maternal systemic infection. Look for red-flag symptoms such as change in the nature of headache, development of focal neurological signs, reduced level of consciousness and atypical headache that may be a hint for different diagnoses. Lying supine for a couple of hours before the EBP may reduce the headache and minimize the volume of CSF in the epidural space that may dilute the injected blood.56 Before beginning with the procedure, secure IV access (for medications if needed). Connect all standard monitoring devices. Two operators are preferred, while one starts the epidural procedure first, the second operator can draw blood. Following aseptic precautions for both epidural and blood draw field, the epidural procedure is initiated. If any difficulty is anticipated in venous blood draw, it can be performed earlier than the epidural. The EBP is performed at the same level of spinal interspace as the initial dural puncture or one space lower, if it is known. In case of multiple needle insertion sites the lowest interspace that was entered is selected as the injected blood usually spreads more cephalad than caudad. Using the loss-of-resistance to air (or saline) technique, epidural space is entered. If anticipating difficulty in identification of epidural space, C-arm radiography or ultrasound guidance may be used. Once the first operator confirms the placement of the needle tip of the 17-gauge epidural needle, the second operator draws approximately 20 mL of autologous blood from the patient in a sterile fashion. The blood is injected slowly over a minute for the formation of a blood patch.5 When performing EBP, around 20 mL of blood is injected; stopping when the patient complains of discomfort or fullness in the back, buttocks, or neck.The volume of blood injected may not determine the strength, thickness and spread of the actual blood clot formed in the epidural space.57
The mean spread of 14 mL of blood is 6 spinal segments cephalad and 3 segments caudad regardless of the bevel direction of Tuohy needle.31 Headache symptoms usually improve soon after the completion of the procedure. The patient is advised to lie in a flat position (maximum 30 degree angle) for a couple of hours with minimal movement, and when found appropriate the patient may resume normal activities.56 They are instructed to avoid lifting, valsalva maneuvers (e.g., straining with bowel movement), and flight journey for one or 2 days after the procedure, to reduce the risk of EBP disruption. Laxatives are prescribed to avoid constipation that is usually associated with opioids prescribed for headache. Twisting and bending need to be avoided and the back has to be kept straight. These strategies may reduce the risk of headache recurrence, but there is insufficient evidence to support this recommendation.56 It is to be kept in mind that sudden analgesic medication discontinuation can result in rebound headache phenomenon and tapering of analgesics is a better choice.3 Women remaining in the hospital are to be reviewed on a daily basis until discharged or symptoms subside. Women discharged home on the same day of procedure need to be contacted the next day. All patients, following an EBP, need to be reviewed by an Anesthetist within four hours of the procedure.56
Complications
Backache due to local nerve root irritation is probably the most common complication25 and may occur in 50% of women. Although it may be present for several days, the severity usually decreases within few days, with resolution for most women by four weeks.56 The incidence of chronic back pain was also found to be higher in patients who had dural punctures and may be part of a chronic postdural puncture syndrome; however, treatment with an EBP does not increase the likelihood of chronic back pain.4 Accidental injection of blood intrathecally can cause major complications which include arachnoiditis or meningitis (blood borne infection), neurological deficits such as cauda equina syndrome or permanent nerve damage. Other complications such as facial nerve paralysis, spinal subdural hematoma (following large volume injections), dizziness, tinnitus, vertigo, and ataxia have also been reported in the literature. Intraventricular blood and PNC after an EBP were reported, both resolved spontaneously without treatment.25
Care must be taken while injecting blood during EBP, especially in patients with CNS issues like Multiple Sclerosis. It is always prudent to inject lesser volumes of blood and slow injection in the epidural space.
Repeated epidural blood patch
Once the causes of headache have been ruled out, a repeat EBP may be performed. Wherever obstetric PDPH is the likely cause or diagnosis and previous EBP has produced reduction in the symptoms, but subsequently return of headache occurs, a second EBP (usually 24-48 hours after the first one) may be offered as it is likely to be of benefit. Wherever an EBP has resulted in no resolution of headache, or if the diagnosis of obstetric PDPH may be doubtful, or there is a change in nature of headache, multispeciality comprehensive discussion should take place before a second EBP is performed. If two EBPs have failed to relieve symptoms, differential diagnosis for headache must be considered and involvement of other related specialties is recommended before performing a third EBP. Evidence is lacking to state the optimum timing for efficacy and safety of a repeat EBP.56
Epidural administration
Epidural saline injections are thought to work by increasing intra cranial pressure (ICP) thereby reducing traction on pain-sensitive structures which cause PDPH. Epidural saline bolus administration may improve symptoms but the effect is usually transient.3 Normal saline is more prone to dissipate from tissue planes and get reabsorbed compared to blood, due to which recurrence of headache is possible. Repeat caudal injections may avoid this problem. A study of the effect of four times repeated caudal saline injection in sessions of 1-2 times a day for 1-2 days (50 to 220 mL) showed that the severity of PDPH was reduced in the majority of patients with each injection of saline, though the headaches were not completely eliminated.59
The use of an epidural hydroxyethyl starch, gelatine or dextran injection may be a suitable alternative for treatment of postdural puncture headache if EBP is contraindicated.60-63 Fibrin glue is another alternative agent to blood that has been proposed to repair dural perforation.25 The successful use of fibrin glue 3-5 mL injected through an epidural needle in the treatment of PDPH and headache associated with spontaneous intracranial hypotension has been reported. There is currently insufficient evidence to recommend the use of epidural dextran, HES, gelatin or fibrin glue in the treatment of obstetric PDPH.3
Acupuncture
Acupuncture has been found to be an easy to apply therapeutic alternative with no significant risks before the consideration for invasive techniques such as EBP. Although the mechanism of action is not known, it has been proposed that it may promote release of endorphins and relieve the muscle spasm.3,64
Greater occipital nerve block and trigger point infiltration
The afferent somatic innervation of most of the occipital region gets interrupted with blocking of greater occipital nerve. This method is basically a symptom-oriented approach to the treatment of PDPH.
Sphenopalatine ganglion blocks
Parasympathetic flow to the cerebral vasculature can be interrupted by blocking the sphenopalatine ganglion. This blockade resists the excessive vasodilation induced by the CSF leak and as the vessels return to their normal diameter, symptoms get relieved.2 Repeat blocks may be needed on more than one occasion.3 The emergency medicine literature suggests that sphenopalatine ganglion block should be the first-line treatment of headache after dural puncture because it is less invasive with reasonable success.39
Surgery
There are rare reports of curative surgical closure of a dural puncture for intractable PDPH. In one case, the interval between dural puncture and surgery was 5 years.
Conclusions
The incidence of UDP is about 1% and it is relatively more common in labouring women. PDPH develops in 60%-80% after UDP, which is very distressing to the patient. PDPH can cause pain and disability during the important time surrounding childbirth. It can also drive up healthcare costs by increasing the length of hospital stay for chronic headache and back pain. EBP is considered the gold standard for the management of PDPH. Active management of PDPH is recommended in a parturient. Proper scheduling of analgesics and supporting the patient psychologically as she cares for her newborn is needed. If the intensity of headache is severe, additional agents, such as caffeine or SPG block may be considered, or one may directly proceed with an EBP. Epidural administration of saline or dextran (fluids other than blood) may be considered if autologous EBP is contraindicated or if an EBP procedure fails.
Acknowledgments
Authors would acknowledge Zahra Yousefi for her assistance in performing this study.
Competing Interests
Authors declare no conflict of interests.
Ethical Approval
Not applicable.
References
- Apfel CC, Saxena A, Cakmakkaya OS, Gaiser R, George E, Radke O. Prevention of post-dural puncture headache after accidental dural puncture: a quantitative systematic review. Br J Anaesth 2010; 105(3):255-63. doi: 10.1093/bja/aeq191 [Crossref] [ Google Scholar]
- Xavier J, Pinho S, Silva J, Nunes CS, Cabido H, Fortuna R. Post-dural puncture headache in the obstetric population: a new approach?. Reg Anesth Pain Med 2020; 45(5):373-6. doi: 10.1136/rapm-2019-101053 [Crossref] [ Google Scholar]
- Russell R, Laxton C, Lucas DN, Niewiarowski J, Scrutton M, Stocks G. Treatment of obstetric post-dural puncture headache Part 1: conservative and pharmacological management. Int J Obstet Anesth 2019; 38:93-103. doi: 10.1016/j.ijoa.2018.12.006 [Crossref] [ Google Scholar]
- Webb CA, Weyker PD, Zhang L, Stanley S, Coyle DT, Tang T. Unintentional dural puncture with a Tuohy needle increases risk of chronic headache. Anesth Analg 2012; 115(1):124-32. doi: 10.1213/ANE.0b013e3182501c06 [Crossref] [ Google Scholar]
- Fattahi Z, Hadavi SM, Sahmeddini MA. Effect of ondansetron on post-dural puncture headache (PDPH) in parturients undergoing cesarean section: a double-blind randomized placebo-controlled study. J Anesth 2015; 29(5):702-7. doi: 10.1007/s00540-015-2000-5 [Crossref] [ Google Scholar]
- Hakim SM. Cosyntropin for prophylaxis against post-dural puncture headache after accidental dural puncture. Anesthesiology 2010; 113(2):413-20. doi: 10.1097/ALN.0b013e3181dfd424 [Crossref] [ Google Scholar]
- Al-Metwalli RR. Epidural morphine injections for prevention of post-dural puncture headache. Anaesthesia 2008; 63(8):847-50. doi: 10.1111/j.1365-2044.2008.05494.x [Crossref] [ Google Scholar]
- Riveros Perez E, Sanchez MG, Rocuts A, Jimenez E. Use of a triple prophylactic strategy to prevent post-dural puncture headache: an observational study. Cureus 2020; 12(2):e7052. doi: 10.7759/cureus.7052 [Crossref] [ Google Scholar]
- Charsley MM, Abram SE. The injection of intrathecal normal saline reduces the severity of post-dural puncture headache. Reg Anesth Pain Med 2001; 26(4):301-5. doi: 10.1053/rapm.2001.22584 [Crossref] [ Google Scholar]
- Okpala BC, Eleje GU, Ikechebelu JI, Ofojebe CJ, Ejikeme TB, Nwachukwu CE. A double-blind placebo-controlled trial on effectiveness of prophylactic dexamethasone for preventing post-dural puncture headache after spinal anesthesia for cesarean section. J Matern Fetal Neonatal Med 2022; 35(17):3407-12. doi: 10.1080/14767058.2020.1818719 [Crossref] [ Google Scholar]
- Yang CJ, Chen T, Ni X, Yu WY, Wang W. Effect of pre-administration with aminophylline on the occurrence of post-dural puncture headache in women undergoing caesarean section by combined spinal-epidural anaesthesia. J Int Med Res 2019; 47(1):420-6. doi: 10.1177/0300060518803231 [Crossref] [ Google Scholar]
- Stein MH, Cohen S, Mohiuddin MA, Dombrovskiy V, Lowenwirt I. Prophylactic vs therapeutic blood patch for obstetric patients with accidental dural puncture--a randomised controlled trial. Anaesthesia 2014; 69(4):320-6. doi: 10.1111/anae.12562 [Crossref] [ Google Scholar]
- Yousefshahi F, Rahat Dahmardeh A, Khajavi M, Najafi A, Khashayar P, Barkhordari K. Effect of dexamethasone on the frequency of post-dural puncture headache after spinal anesthesia for cesarean section: a double-blind randomized clinical trial. Acta Neurol Belg 2012; 112(4):345-50. doi: 10.1007/s13760-012-0065-6 [Crossref] [ Google Scholar]
- Faridi Tazeh-Kand N, Eslami B, Ghorbany Marzony S, Abolhassani R, Mohammadian K. Injection of intrathecal normal saline in decreasing post-dural puncture headache. J Anesth 2014; 28(2):206-9. doi: 10.1007/s00540-013-1683-8 [Crossref] [ Google Scholar]
- Lee SI, Sandhu S, Djulbegovic B, Mhaskar RS. Impact of spinal needle type on post-dural puncture headache among women undergoing cesarean section surgery under spinal anesthesia: a meta-analysis. J Evid Based Med 2018; 11(3):136-44. doi: 10.1111/jebm.12311 [Crossref] [ Google Scholar]
- Zhao G, Song G, Liu J. Efficacy of pharmacological therapies for preventing post-dural puncture headaches in obstetric patients: a Bayesian network meta-analysis of randomized controlled trials. BMC Pregnancy Childbirth 2023; 23(1):215. doi: 10.1186/s12884-023-05531-7 [Crossref] [ Google Scholar]
- Wu CL, Rowlingson AJ, Cohen SR, Michaels RK, Courpas GE, Joe EM. Gender and post-dural puncture headache. Anesthesiology 2006; 105(3):613-8. doi: 10.1097/00000542-200609000-00027 [Crossref] [ Google Scholar]
- Angle P, Thompson D, Halpern S, Wilson DB. Second stage pushing correlates with headache after unintentional dural puncture in parturients. Can J Anaesth 1999; 46(9):861-6. doi: 10.1007/bf03012976 [Crossref] [ Google Scholar]
- Russell TW, Rosc AR, McShane FJ. The incidence of post-dural puncture headache in the obese parturient compared to the non-obese parturient after an accidental dural puncture: a systematic review protocol. JBI Evid Synth 2020; 18(6):1320-5. doi: 10.11124/jbisrir-d-19-00037 [Crossref] [ Google Scholar]
- Song J, Breidenbach K, Duong AL, Zhang S, Joseph V. Impact of migraine headaches and depression/anxiety on the incidence of post-dural puncture headache during postpartum course. Australas Med J 2018; 11(3):178-85. doi: 10.21767/amj.2018.3346 [Crossref] [ Google Scholar]
- Dodge HS, Ekhator NN, Jefferson-Wilson L, Fischer M, Jansen I, Horn PS. Cigarette smokers have reduced risk for post-dural puncture headache. Pain Physician 2013; 16(1):E25-30. [ Google Scholar]
- Omole OB, Ogunbanjo GA. Post-dural puncture headache: evidence-based review for primary care. S Afr Fam Pract 2015; 57(4):241-6. doi: 10.1080/20786190.2015.1014154 [Crossref] [ Google Scholar]
- Ahmed SV, Jayawarna C, Jude E. Post lumbar puncture headache: diagnosis and management. Postgrad Med J 2006; 82(973):713-6. doi: 10.1136/pgmj.2006.044792 [Crossref] [ Google Scholar]
- Zhang D, Chen L, Chen X, Wang X, Li Y, Ning G. Lower incidence of post-dural puncture headache using Whitacre spinal needles after spinal anesthesia: a meta-analysis. Headache 2016; 56(3):501-10. doi: 10.1111/head.12745 [Crossref] [ Google Scholar]
- Kracoff SL, Kotlovker V. Post-dural puncture headache—review and suggested new treatment. Open J Anesthesiol 2016; 6(9):148-63. doi: 10.4236/ojanes.2016.69024 [Crossref] [ Google Scholar]
- Parker RK, White PF. A microscopic analysis of cut-bevel versus pencil-point spinal needles. Anesth Analg 1997; 85(5):1101-4. doi: 10.1097/00000539-199711000-00025 [Crossref] [ Google Scholar]
- Depaulis C, Steer N, Garessus L, Chassard D, Aubrun F. Evaluation of the effectiveness and tolerance of tetracosactide in the treatment of post-dural puncture headaches (ESYBRECHE): a study protocol for a randomised controlled trial. Trials 2020; 21(1):55. doi: 10.1186/s13063-019-4015-y [Crossref] [ Google Scholar]
- Nguyen DT, Walters RR. Standardizing management of post-dural puncture headache in obstetric patients: a literature review. Open J Anesthesiol 2014; 4(10):244. doi: 10.4236/ojanes.2014.410037 [Crossref] [ Google Scholar]
- Norris MC, Leighton BL, DeSimone CA. Needle bevel direction and headache after inadvertent dural puncture. Anesthesiology 1989; 70(5):729-31. doi: 10.1097/00000542-198905000-00002 [Crossref] [ Google Scholar]
- Corbey MP, Bach AB, Lech K, Frørup AM. Grading of severity of post-dural puncture headache after 27-gauge Quincke and Whitacre needles. Acta Anaesthesiol Scand 1997; 41(6):779-84. doi: 10.1111/j.1399-6576.1997.tb04783.x [Crossref] [ Google Scholar]
- Alam M, Raheen M, Iqbal K, Chowdhury M. Headache following spinal anaesthesia: a review on recent update. J Bangladesh Coll Phys Surg 2011; 29(1):32-40. [ Google Scholar]
- Strupp M, Brandt T, Müller A. Incidence of post-lumbar puncture syndrome reduced by reinserting the stylet: a randomized prospective study of 600 patients. J Neurol 1998; 245(9):589-92. doi: 10.1007/s004150050250 [Crossref] [ Google Scholar]
- Sadashivaiah J, McLure H. 18-G Tuohy needle can reduce the incidence of severe post-dural puncture headache. Anaesthesia 2009; 64(12):1379-80. doi: 10.1111/j.1365-2044.2009.06141_14.x [Crossref] [ Google Scholar]
- Zorrilla-Vaca A, Makkar JK. Effectiveness of lateral decubitus position for preventing post-dural puncture headache: a meta-analysis. Pain Physician 2017; 20(4):E521-9. [ Google Scholar]
- Candido KD, Stevens RA. Post-dural puncture headache: pathophysiology, prevention and treatment. Best Pract Res Clin Anaesthesiol 2003; 17(3):451-69. doi: 10.1016/s1521-6896(03)00033-8 [Crossref] [ Google Scholar]
- Ready LB, Cuplin S, Haschke RH, Nessly M. Spinal needle determinants of rate of transdural fluid leak. Anesth Analg 1989; 69(4):457-60. [ Google Scholar]
- Thejaswini HS, Honnanavar K, Rao R. Comparison of incidence of PDPH between two different gauges of Whitacre spinal needles in patients undergoing caesarean section. Int J Med Anesthesiol 2019; 2(2):140-4. doi: 10.33545/26643766.2019.v2.i2b.40 [Crossref] [ Google Scholar]
- Sprigge JS, Harper SJ. Accidental dural puncture and post-dural puncture headache in obstetric anaesthesia: presentation and management: a 23-year survey in a district general hospital. Anaesthesia 2008; 63(1):36-43. doi: 10.1111/j.1365-2044.2007.05285.x [Crossref] [ Google Scholar]
- Gaiser RR. Post-dural puncture headache: an evidence-based approach. Anesthesiol Clin 2017; 35(1):157-67. doi: 10.1016/j.anclin.2016.09.013 [Crossref] [ Google Scholar]
- Baraz R, Collis RE. The management of accidental dural puncture during labour epidural analgesia: a survey of UK practice. Anaesthesia 2005; 60(7):673-9. doi: 10.1111/j.1365-2044.2005.04222.x [Crossref] [ Google Scholar]
- Agerson AN, Scavone BM. Prophylactic epidural blood patch after unintentional dural puncture for the prevention of post-dural puncture headache in parturients. Anesth Analg 2012; 115(1):133-6. doi: 10.1213/ANE.0b013e31825642c7 [Crossref] [ Google Scholar]
- Heesen M, Klöhr S, Rossaint R, Walters M, Straube S, van de Velde M. Insertion of an intrathecal catheter following accidental dural puncture: a meta-analysis. Int J Obstet Anesth 2013; 22(1):26-30. doi: 10.1016/j.ijoa.2012.10.004 [Crossref] [ Google Scholar]
- Modir H, Moshiri E, Modir A, Modir A, Mohammadbeigi A. The preventive effects of oral caffeine and melatonin on headache after spinal anesthesia for lower limb surgery: a double-blinded, randomized clinical trial. Indian Anaesth forum 2020; 21(1):50-5. doi: 10.4103/TheIAForum.TheIAForum_85_19 [Crossref] [ Google Scholar]
- Al Amri ZS, Al-Jadidi A, Khan RM, Kaul N. Retrospective analysis of clinical efficacy of protocol-based management of post-dural puncture headache in patients undergoing cesarean section under spinal anesthesia. Indian J Pain 2017; 31(1):18-22. doi: 10.4103/ijpn.ijpn_3_17 [Crossref] [ Google Scholar]
- Wagner Y, Storr F, Cope S. Gabapentin in the treatment of post-dural puncture headache: a case series. Anaesth Intensive Care 2012; 40(4):714-8. doi: 10.1177/0310057x1204000420 [Crossref] [ Google Scholar]
- Huseyinoglu U, Huseyinoglu N, Hamurtekin E, Aygun H, Sulu B. Effect of pregabalin on post-dural-puncture headache following spinal anesthesia and lumbar puncture. J Clin Neurosci 2011; 18(10):1365-8. doi: 10.1016/j.jocn.2011.02.029 [Crossref] [ Google Scholar]
- Dogan Erol D. The effect of oral gabapentin on post-dural puncture headache. Acute Pain 2006; 8(4):169-73. doi: 10.1016/j.acpain.2006.08.042 [Crossref] [ Google Scholar]
- Kassim DY, Esmat IM. Comparative study between hydrocortisone and mannitol in treatment of post-dural puncture headache: a randomized double-blind study. Egypt J Anaesth 2016; 32(3):357-63. doi: 10.1016/j.egja.2016.05.004 [Crossref] [ Google Scholar]
- Gherghina VI, Nicolae G, Cîndea I, Balcan A, Popescu R, Costea D. Effect of intravenous methylprednisolone in the treatment of post-dural puncture headache: a double blind controlled clinical study: 8AP3-9. Eur J Anaesthesiol 2013; 30:124. [ Google Scholar]
- Wu C, Guan D, Ren M, Ma Z, Wan C, Cui Y. Aminophylline for treatment of post-dural puncture headache: a randomized clinical trial. Neurology 2018; 90(17):e1523-9. doi: 10.1212/wnl.0000000000005351 [Crossref] [ Google Scholar]
- Utku Yıldırım H, Bakır M, Rumeli Atıcı Ş. Evaluation of theophylline efficiency in post-dural puncture headache. J Anesth 2020; 28(4):247-54. doi: 10.5222/jarss.2020.74436 [Crossref] [ Google Scholar]
- Stannard D. Epidural blood patching for preventing and treating post-dural puncture headache. J Perianesth Nurs 2011; 26(6):411-2. doi: 10.1016/j.jopan.2011.09.002 [Crossref] [ Google Scholar]
- Ho KY, Gan TJ. Management of persistent post-dural puncture headache after repeated epidural blood patch. Acta Anaesthesiol Scand 2007; 51(5):633-6. doi: 10.1111/j.1399-6576.2007.01283.x [Crossref] [ Google Scholar]
- Kawaguchi M, Hashizume K, Watanabe K, Inoue S, Furuya H. Fluoroscopically guided epidural blood patch in patients with post-dural puncture headache after spinal and epidural anesthesia. J Anesth 2011; 25(3):450-3. doi: 10.1007/s00540-011-1135-2 [Crossref] [ Google Scholar]
- Pouskoulas CD, Taub E, Ruppen W. Successful treatment of post-dural puncture headache with surgical dura repair two years after spinal anesthesia. Cephalalgia 2013; 33(15):1269-71. doi: 10.1177/0333102413490348 [Crossref] [ Google Scholar]
- Russell R, Laxton C, Lucas DN, Niewiarowski J, Scrutton M, Stocks G. Treatment of obstetric post-dural puncture headache Part 2: epidural blood patch. Int J Obstet Anesth 2019; 38:104-18. doi: 10.1016/j.ijoa.2018.12.005 [Crossref] [ Google Scholar]
- Booth JL, Pan PH, Thomas JA, Harris LC, D’Angelo R. A retrospective review of an epidural blood patch database: the incidence of epidural blood patch associated with obstetric neuraxial anesthetic techniques and the effect of blood volume on efficacy. Int J Obstet Anesth 2017; 29:10-7. doi: 10.1016/j.ijoa.2016.05.007 [Crossref] [ Google Scholar]
- Gurudatt CL. Unintentional dural puncture and post-dural puncture headache-can this headache of the patient as well as the anaesthesiologist be prevented?. Indian J Anaesth 2014; 58(4):385-7. doi: 10.4103/0019-5049.138962 [Crossref] [ Google Scholar]
- Abdulla S, Abdulla W, Eckhardt R. Caudal normal saline injections for the treatment of post-dural puncture headache. Pain Physician 2011; 14(3):271-9. [ Google Scholar]
- Aldrete JA. Persistent post-dural puncture headache treated with epidural infusion of dextran. Headache 1994; 34(5):265-7. doi: 10.1111/j.1526-4610.1994.hed3405265.x [Crossref] [ Google Scholar]
- Sun S, Huang SQ. Epidural injection of hydroxyethyl starch in the management of post-dural puncture headache: a case series. Int J Clin Exp Med 2015; 8(5):8254-8. [ Google Scholar]
- Ambesh SP, Kumar A, Bajaj A. Epidural gelatin (Gelfoam®) patch treatment for post-dural puncture headache. Anaesth Intensive Care 1991; 19(3):444-7. doi: 10.1177/0310057x9101900325 [Crossref] [ Google Scholar]
- Vassal O, Baud MC, Bolandard F, Bonnin M, Vielle E, Bazin JE. Epidural injection of hydroxyethyl starch in the management of post-dural puncture headache. Int J Obstet Anesth 2013; 22(2):153-5. doi: 10.1016/j.ijoa.2013.01.003 [Crossref] [ Google Scholar]
- Dietzel J, Witstruck T, Adler S, Usichenko TI. Acupuncture for treatment of therapy-resistant post-dural puncture headache: a retrospective case series. Br J Anaesth 2013; 111(5):847-9. doi: 10.1093/bja/aet369 [Crossref] [ Google Scholar]