J Res Clin Med. 12:16.
doi: 10.34172/jrcm.33401
Original Article
Accuracy of mitral valve annulus measurements by two-and three-dimensional transesophageal echocardiography, compared to intraoperative measurements in patients undergoing mitral valve replacement surgery
Mehrnoush Toufan Tabrizi Conceptualization, Investigation, Methodology, Project administration, Validation, 
Elnaz Javanshir Data curation, Formal analysis, Investigation, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, * 
Naser Khezerlouy-Aghdam Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing, 
Author information:
Cardiovascular Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Transthoracic echocardiography (TTE) is the most beneficial diagnostic modality for mitral valve (MV) diseases. However, it is not accurate enough for MV annular sizing. Recent evidence has demonstrated the superiority of transesophageal echocardiography (TEE) over TTE. The present study aimed to evaluate the accuracy of two-dimensional TEE (2D-TEE) and three-dimensional TEE (3D-TEE) in preoperative measuring of MV size and specifications.
Methods:
This cross-sectional study evaluated patients with mitral valve replacement (MVR) surgery at Shahid Madani Heart Center of Tabriz university of Medical Sciences, Tabriz, Iran, in 2020. Thirty-eight patients admitted for MVR surgery were enrolled in this study. Demographic, electrocardiogram (ECG), and echocardiographic studies -including TTE, 2D, and 3D TEE- were performed preoperative for each patient. The accurate size and the specifications of the studied structure were measured by a surgical gauge caliper during the surgery, which was considered the gold standard value. The correlations between obtained parameters were evaluated using Pearson’s correlation coefficient. The data obtained from individual were entered into the SPSS software. Significance was set at P<0.05.
Results:
The mean age of patients was 55.89±11.81 years, and 65.8% were male. Most patients (68.4%) had sinus rhythm. The strongest correlation with the gold standard was observed in mitral annulus circumference measured by the three-dimensional multiplanar reconstruction (3D MPR) method in all patients (r=0.758, P<0.001), [(r=0.723, P<0.001) for patients with sinus rhythm, and (r=0.825, P=0.001) for patients with atrial fibrillation (AF)]. The minor difference between the echocardiographic measurements and the surgical rule was related to the short axis (SA) in the 3D MPR method (3.3 mm).
Conclusion:
This study illustrated a high correlation between 3D-MPR TEE measured MV annulus and intra-operative measurements using a surgical gauge caliper as the gold standard.
Keywords: Echocardiography, Mitral valve, Mitral annuloplasty, Transesophageal
Copyright and License Information
© 2024 The Authors.
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This research did not receive any specific grant or funding of any sort.
Introduction
Valvular heart disease (VHD) is characterized by distorted intra-cardiac valve structure(s), resulting in diminished physiologic cardiac functioning. It causes a prominent impairment in patient’s quality of life and imposes high annual costs on healthcare system.1 Amongst VHDs, mitral valve (MV) disease is the most common in the developed countries.2 The culprit is that the MV’s complex structure makes it more vulnerable to insults.3
Surgical repair of the diseased valve will improve the outcome in some cases.4 Meanwhile, choosing a proper method, case selection, and accurate timing for intervention are crucial for reaching a desirable outcome. The latter is unachievable unless the physicians are able to obtain thorough and precise information on MV apparatus anatomy and function prior to the surgery.5,6 Transthoracic echocardiography (TTE) is the primarily used modality for patient evaluation in this regard. The type of lesion, the severity of the disease, the functional status of the valve, and the burden of the disease on overall cardiac function are somehow evaluable by TTE.7 However, transesophageal echocardiography (TEE) has higher sensitivity and can provide more accurate information compared to TTE. Therefore, the TEE study is recommended for all patients with VHD who do not have any contraindications.8
On the other hand, considering the different modes of echocardiographic studies, three-dimensional (3D) images are superior to two-dimensional (2D) and provide comprehensive data from the study object (MV apparatus in this case).9-11 Thus, 3D TEE study has been suggested as a complementary modality for all patients with MV disease. It has been stated that the 3D TEE can provide valuable preoperative information for both the surgeon and the cardiologist. 6,9 However, a few comparative studies have been conducted so far in this regard.12 Since the precise measuring of the valve annulus is crucial before MV replacement surgery,13 besides the lack of sufficient studies comparing the pros and cons of the preoperative 3D TEE over the conventional echocardiographic studies; we aimed to conduct this study. The primary objective of the present study is to find out which echocardiographic methods (2D and 3D) represents more accurate results in measuring MV annulus size and specifications.
Methods
This cross-sectional study was conducted at Shahid Madani Heart Center of Tabriz University of Medical Sciences, Tabriz, Iran, in 2020. Thirty-eight patients over 18 years with MV disease who were selected for mitral valve replacement (MVR) surgery were enrolled in this study. The indication for surgery was determined according to relative guidelines, regardless of the present study’s design. Individuals were selected using the convenience sampling method and based on the inclusion criteria. All patients had undergone MVR surgery due to MV regurgitation, valve stenosis, or endocarditis as a direct consequence of MV disorder. Following recruitment, the demographic data of the individuals, including age, body surface area (BSA), and the etiology of their MV disease, were recorded on each patient’s chart. BSA was calculated using the BSA calculator. Each patient was stratified into one of the following groups of: rheumatic, functional, prolaptic, degenerative, and infective valve disease according to the etiology of their disease. Combined pathologies were also labeled as a separate etiological classification.
Written informed consent was obtained from all participants, ensuring their information could be used for medical research. The collected information and the results of the statistical analysis remained confidential.
Afterward, electrocardiogram (ECG), as well as conventional 2D TTE studies, were performed for all patients. Left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular ejection fraction (LVEF), left atrial volume index (LAVI), and pulmonary artery pressure (PAP) were measured, and charted for each individual. Further on, all patients were studied by 2D TEE in order to measure MV annulus. The measurements were performed at three echocardiographic views (1) Mediolateral (Zero-degree angle), (2) Commissural (75-to-90-degree angle), and (3) Antero-posterior (120-degree angle). The mid-diastolic size of the valve annulus, which is the largest physiological diameter, was the measure of interest for the investigators. At the final stage, 3D TEE in zoom mode and mid-esophageal level (60-to-75-degree angle) was performed to fulfill the pre-operative survey of the study participants. All acquired data were then summoned and inserted into QLAB software for offline analysis.
MV annulus diameter -at both short and long axis- as well as MV surface area and circumference were calculated for each patient using 3D multiplanar reconstruction and 3D direct methods. The mean of the three and five separate beats measures was considered the final value in patients with normal sinus rhythm and atrial fibrillation (AF), respectively.
All echocardiographic studies were performed utilizing a Philips® EPIQ 7 ultrasound machine. Mathematical values were calculated using the QLAB software, following the adjustment of the depth and gain of the obtained images (Figure 1). Patients with severe mitral annulus calcification and/or inadequate quality of echocardiographic images were excluded from the study.
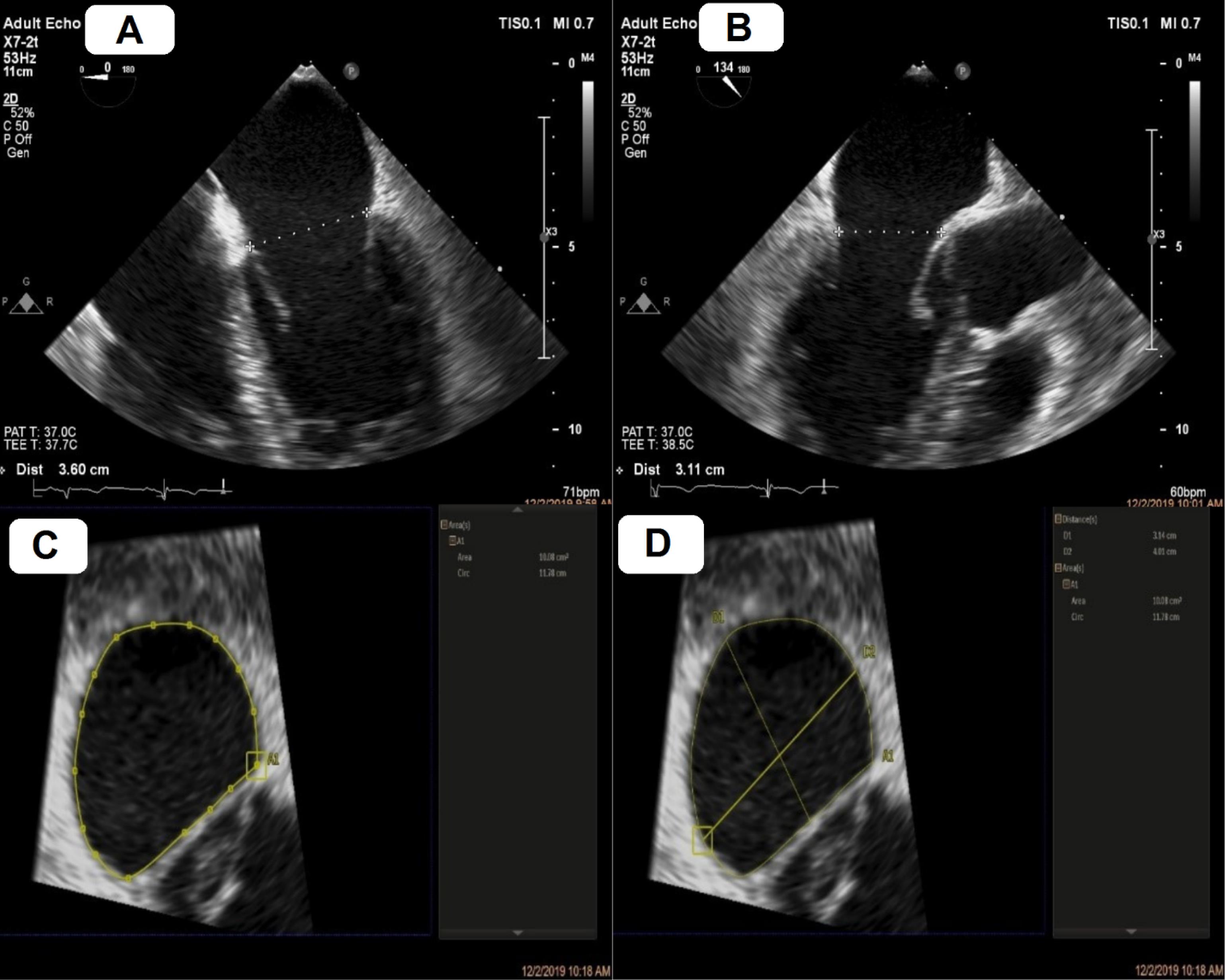
Figure 1.
Mitral annulus sizing using two– and three–dimensional transesophageal echocardiography (2D and 3D TEE) software. A, mediolateral annulus by 2D TEE. B, anteroposterior annulus by 2D TEE. C, annulus area and circ by 3D multiplanar reconstruction. D, Long axis (LA) and short axis (SA) by 3D multiplanar reconstruction.
.
Mitral annulus sizing using two– and three–dimensional transesophageal echocardiography (2D and 3D TEE) software. A, mediolateral annulus by 2D TEE. B, anteroposterior annulus by 2D TEE. C, annulus area and circ by 3D multiplanar reconstruction. D, Long axis (LA) and short axis (SA) by 3D multiplanar reconstruction.
In order to address the investigator bias, all echocardiographic studies were carried out by two highly trained echocardiologists. Both observers were kept blind to each other’s results. Inter-observer agreement (IOA) was then calculated using the kappa coefficient.
Intra-operative MV annulus size was considered the gold standard in order to compare the echocardiographic results. For this purpose, the mitral annulus was measured at its maximal diameter on an unloaded state using a surgical gauge caliper. Mitral annulus area (MAA) and mitral annulus circumference (MAC) were calculated based on the obtained diameter.
First, the researchers explained the design and objective of the study to the eligible participants and asked them to read and sign the written informed consent if they wish to participate in the study. Patients were told that they are free to leave the study at any stage if they were reluctant to carry on with the study team.
Statistical analysis
The data were analyzed using IBM SPSS Statistics for Windows version 22.0 (IBM Corp. 2013. Armonk, NY: IBM Corp). The descriptive analysis was expressed as numbers, frequencies, and mean ± standard deviation (SD). The categorical variables were compared using the Chi-square test. The normal distribution of data was evaluated using the Shapiro-Wilk test, which showed normal distribution of all numeric variables. Therefore, the statistical associations between the values were compared using paired t-test and Pearson’s correlation coefficient. Also, the correlation was calculated according to the patients’ BSA. A P < 0.05 was considered to be statistically significant.
Results
A total of 38 patients diagnosed with end-stage mitral regurgitation (MR) or mitral stenosis (MS), according to the American Heart Association and American College of Cardiology (AHA/ACC) guidelines, completed this study. All participants were successfully treated with metallic MVR surgery later in the course of the study. Coronary bypass surgery grafting (CABG) was performed simultaneously for 10 out of 38 study participants.
Since the echocardiographic studies were performed by two independent echocardiologists, the IOA was evaluated which revealed a kappa value of 0.87. Besides, data were normally distributed, according to the Shapiro-Wilk statistical analysis.
Regarding the patients’ demographic information, the mean age and BSA of patients were 55.89 ± 11.81 years and 1.81 ± 0.17 m2, respectively; and 13 (34.2%) were female. Considering the etiology of the valvular disease, rheumatic valve disease with the incidence of 21 out of 38 patients (55.3%) was on top of our list followed by functional (13.2%, n = 5), prolaptic (10.5%, n = 4), degenerative-functional (7.9%, n = 3), degenerative (5.3%, n = 2), prolaptic-infective endocarditis (5.3%, n = 2), and infective endocarditis (2.6%, n = 1)
According to the ECG data, most patients had sinus rhythm (68.4% n = 26). Table 1 depicts the data regarding the mitral annulus diameter (MAD), MAA, and MAC stratified according to the measuring method.
Table 1.
The measurements of transthoracic echocardiographic, two– and three–dimensional transesophageal echocardiography
|
Type of test
|
Measurements
|
Mean±SD
|
| Two–dimensional transesophageal echocardiography |
Mediolateral annulus, mm |
38.27 ± 4.03 |
| Commissure–commissure annulus, mm |
37.51 ± 4.39 |
| Anteroposterior annulus, mm |
36.93 ± 4.07 |
| Three–dimensional transesophageal echocardiography |
LA (3D MPR), mm |
39.38 ± 4.61 |
| SA (3D MPR), mm |
31.91 ± 3.19 |
| Area (3D MPR), cm2 |
10.26 ± 2.1 |
| Circ (3D MPR), cm |
11.82 ± 1.22 |
| LA (3D Direct), mm |
37.92 ± 4.56 |
| SA (3D Direct), mm |
31.54 ± 3.62 |
| Area (3D Direct), cm2 |
9.92 ± 1.98 |
| Circ (3D Direct) cm |
11.48 ± 1.14 |
| Echocardiographic measurements |
LVEDD, mm |
51.61 ± 8.61 |
| LVEDDI, mm/m2 |
28.41 ± 4.54 |
| LVESD, mm |
36.31 ± 9.71 |
| LVESDI, mm/m2 |
19.91 ± 4.95 |
| EF% |
46.93 ± 8.29 |
| LAVI, cc/m2 |
73.36 ± 36.99 |
| PAP, mmHg |
51.05 ± 22.14 |
Abbreviations: LA; long axis, 3D; three–dimensional, MPR; multiplanar reconstruction; SA; short axis, Circ; circumference, LVEDD; left ventricular end-diastolic diameter, LVEDDI; left ventricular end-diastolic dimension index, LVESD; left ventricular end-systolic dimension, LVESDI; left ventricular end-systolic dimension index, EF; ejection fraction, LAVI; left atrial volume index, PAP; pulmonary artery pressure.
The correlation of the data obtained from 2D and 3D TEE compared to the surgical gold standard is displayed in both Table 2 and Figure 2. The strongest correlation with the gold standard was observed in the MAC values when it was measured using the 3D MPR method (r = 0.758, P < 0.001). The latter statistical significance was also discretely verifiable within the population of patients with sinus rhythm (r = 0.723, P < 0.001) and AF rhythm (r = 0.825, P = 0.001).
Table 2.
The Pearson’s correlation between mitral valve annulus diameters, area, and circumference of two– and three–dimensional transesophageal echocardiography with the surgical ruler
|
Variables
|
Measurements
|
Total
|
Sinus rhythm
|
Atrial fibrillation
|
|
r
|
P
value
|
r
|
P
value
|
r
|
P
value
|
| Diameter |
Mediolateral annulus |
0.477 |
0.001 |
0.440 |
0.01 |
0.407 |
0.168 |
| Commissure–commissure annulus |
0.451 |
0.002 |
0.425 |
0.014 |
0.390 |
0.188 |
| Anteroposterior annulus |
0.468 |
0.001 |
0.452 |
0.008 |
0.287 |
0.341 |
| LA (3D MPR) |
0.493 |
< 0.001 |
0.480 |
0.005 |
0.346 |
0.248 |
| SA (3D MPR) |
0.507 |
< 0.001 |
0.479 |
0.005 |
0.400 |
0.175 |
| LA (3D Direct) |
-0.234 |
0.157 |
0.014 |
0.946 |
-0.766 |
0.006 |
| SA (3D Direct) |
-0.06 |
0.722 |
0.175 |
0.383 |
-0.678 |
0.022 |
| Area |
Area (3D MPR) |
0.587 |
< 0.001 |
0.561 |
0.001 |
0.524 |
0.066 |
| Area (3D Direct) |
-0.188 |
0.258 |
0.034 |
0.867 |
-0.728 |
0.011 |
| Circ |
Circ (3D MPR) |
0.758 |
< 0.001 |
0.723 |
< 0.001 |
0.825 |
0.001 |
| Circ (3D Direct) |
-0.179 |
0.282 |
0.061 |
0.764 |
–0.688 |
0.019 |
Abbreviations: LA; long axis, 3D; three–dimensional, MPR; multiplanar reconstruction; SA; short axis, Circ; circumference.
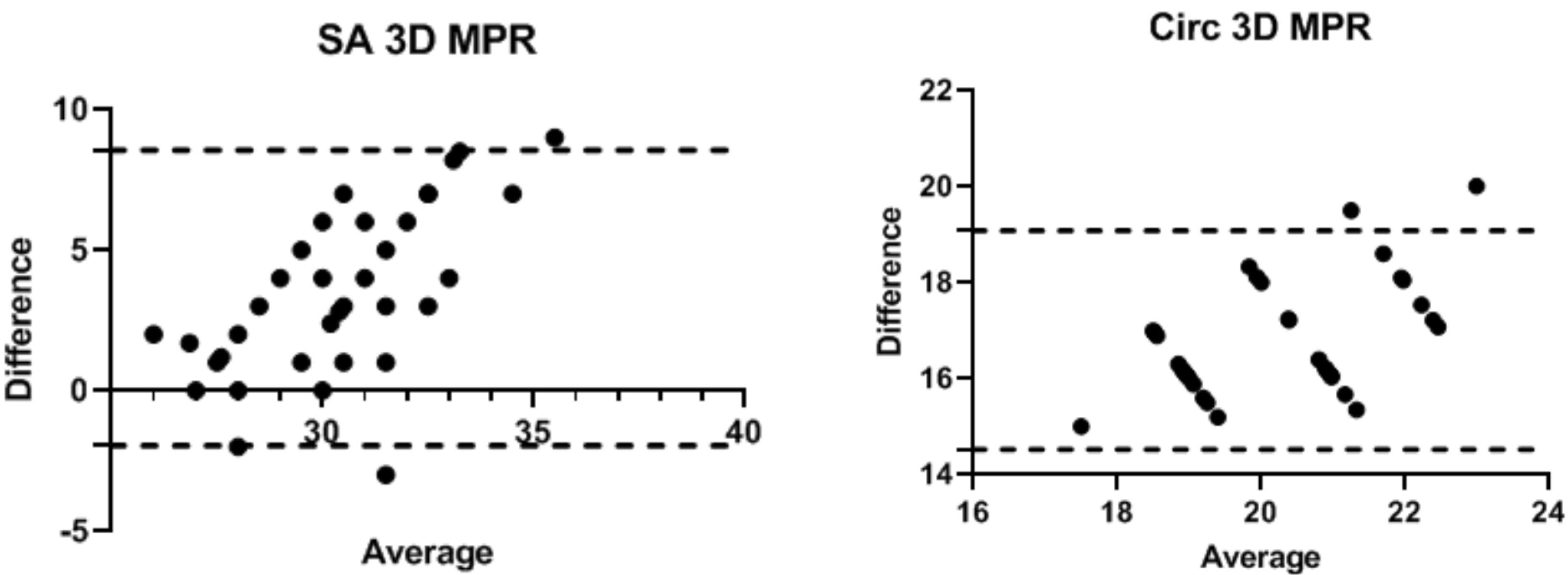
Figure 2.
The correlation of SA 3D MPR and circ 3D MPR with the surgical ruler
.
The correlation of SA 3D MPR and circ 3D MPR with the surgical ruler
However, none of the indices of the 3D direct planimetry were significantly correlated with the surgical gold standard (P > 0.05).
As depicted in Table 3, the mitral annulus values measured by 2D and 3D TEE methods revealed a significant correlation with the surgical gold standard among the patients with sinus rhythm. Meanwhile, apart from the mediolateral annulus size, long-axis diameter of the annulus, and annulus circumference, the other measured values did not show a significant correlation with gold standard observation in AF patients (Table 3).
Table 3.
The Pearson’s correlation between diameters, area, and circumference of two– and three–dimensional transesophageal echocardiography with surgical ruler based on patients’ body surface area
|
Variables
|
Total
|
Sinus rhythm
|
Atrial fibrillation
|
|
r
|
P
value
|
r
|
P
value
|
r
|
P
value
|
| Mediolateral annulus |
0.744 |
0.001 |
0.754 |
0.001 |
0.616 |
0.033 |
| Commissure–commissure annulus |
0.679 |
0.001 |
0.728 |
0.001 |
0.398 |
0.201 |
| Anteroposterior annulus |
0.737 |
0.001 |
0.756 |
0.001 |
0.567 |
0.055 |
| LA (3D MPR) |
0.670 |
0.001 |
0.651 |
0.001 |
0.619 |
0.032 |
| SA (3D MPR) |
0.735 |
0.001 |
0.749 |
0.001 |
0.568 |
0.054 |
| Area (3D MPR) |
0.694 |
0.001 |
0.716 |
0.001 |
0.438 |
0.154 |
| Circ (3D MPR) |
0.788 |
0.001 |
0.824 |
0.001 |
0.590 |
0.043 |
Abbreviations: LA; long axis, 3D; three–dimensional, SA; short axis, Circ; circumference.
According to the data presented in Table 4, the most accurate echocardiographic values compared to the gold standard belong to the SA when they were measured using the 3D MPR method. Other echocardiographic values and their deviation from the gold standard are shown in Table 4. All patients completed this study successfully and uneventfully.
Table 4.
The difference between transesophageal echocardiographic measurements with the surgical ruler
|
Variables
|
Measurements
|
Mean±SD
|
Difference
|
P
value
|
| Diameter |
Surgical ruler |
28.60±1.71 |
- |
|
| LA (3D MPR) |
39.38±4.61 |
-10.78 |
< 0.001 |
| SA (3D MPR) |
31.90±3.19 |
-3.3 |
< 0.001 |
| LA (3D Direct) |
37.92±4.56 |
-9.23 |
< 0.001 |
| SA (3D Direct) |
31.54±3.62 |
-2.85 |
< 0.001 |
| Mediolateral |
38.27±4.03 |
-9.66 |
< 0.001 |
| Commissure–commissure |
37.51±4.39 |
-8.91 |
< 0.001 |
| Anteroposterior |
36.93±4.07 |
-8.32 |
< 0.001 |
| Area |
Surgical ruler |
6.05±1.15 |
- |
- |
| Area (3D MPR) |
10.26±2.10 |
-4.21 |
< 0.001 |
| Circ |
Surgical ruler |
8.98±0.56 |
|
|
| Circ (3D MPR) |
11.82±1.22 |
-2.84 |
< 0.001 |
Abbreviations: LA; long axis, 3D; three–dimensional, MPR; multiplanar reconstruction; SA; short axis, Circ; circumference.
Discussion
The results of this study revealed a positive correlation in the values of MAD, MAA, and MAC, measured by 2D and 3D TEE-MPR methods, compared to the actual numbers obtained following valvectomy. The P value for these observations was less than alpha, hence considered significant. These results express a high diagnostic accuracy for 2D and 3D TEE in order to scrutinize the MV annulus prior to the MVR surgery, which is crucial in terms of patient and device selection, as well as choosing the proper implant size and surgical technique. However, 3D direct planimetry measurements were not precise enough in this regard. This might have happened due to the difficulty in accurately locating the distorted borders of the valve’s annulus in the setting of degenerative and rheumatic valve diseases.
The superiority of the 3D TEE in assessing the MV annulus over the conventional 2D methods has been suggested by several studies. They related their findings to the spatial saddle shape of MV, which is not accurately comprehensible in conventional 2D plan images.9-11,14
Furthermore, 3D TEE has been shown to exhibit MV morphology in high consistency with computed tomography (CT) images that makes it a perfect and safe alternative for CT scans.15 However, the quantitative comparison of diagnostic accuracy of pre-surgical 2D and 3D TEE compared to surgically determined MV parameters has rarely been studied. In a retrospective study on 94 patients with degenerative MV disease undergoing 3D TEE, MAC demonstrated the strongest correlation with the implanted annuloplasty band’s length (r = 0.74).12 The study performed by Calleja and colleagues12 declares a set of results consistent with the present study, which remark the strong correlation between MAC and the gold standard. However, their methods of collecting reference standard and inclusion criteria were different from the present study.1 Another study that compared 2D and 3D TEE results with the intraoperatively obtained measures yields sensitivities of 97.7% and 92.9%, respectively, and a remarkable specificity of 100% for both methods. These results are consistent with the present study confirming a high correlation with intraoperative gold standard value for both 2D and 3D TEE methods.16
Although intraoperative sizing is the conventional and prevalently used method for determining the MV implant size, comprehending this value prior to the surgery can add tremendous leeway for surgeons to properly prepare themselves for the operation.8,15 Therefore, the availability of a method capable of preoperatively and accurately visualizing the MV apparatus, would be valuable in this regard.7,16
In a study by Ender et al, the researchers studied 35 patients with MV diseases who were selected for elective surgical valve repair. According to their results, the correlation between real-time 3D TEE measures performed before the surgery, and the intra-operatively measured actual valve’s ring size was 0.91. In the same way, this correlation for the inter-commissural distance and the height of the anterior MV leaflet were 0.55 and 0.75, respectively. Moreover, the statistical correlation coefficient regarding the relativity of the implanted ring size and the postoperative MV assessment, as well as the intercommissural distance and the height of the anterior MV leaflet were 0.96, 0.92, and 0.79, respectively.17 These results indicate the high statistical correlation running between the real-time 3D TEE and surgically obtained MV measures, consistent with the present study.
The significant impact of the MAD, MAA, and MAC on prognosis of patients with MV disease persuaded us to opt these parameters.18,19 We also suggest further studies focusing on these parameters.
Subgroup analysis in the present study also showed that the observed significant correlation between the MV annulus circumference measured by the 3D MPR method compared to the gold standard, is valid for both groups of patients with sinus and AF rhythm. However, most other statistical correlations were exclusively attainable among patients with sinus rhythm. The recent finding might have happened due to the small sample size of the current study. Though, all correlations remained significant after considering BSA in the subgroup analysis. These results confirm high levels of correlation running between 2D/3D TEE findings and the surgical measurements independent of patients’ BSA and rhythm (in the case of MV annulus circumference), which is contrary to the previous studies.19-22
Regarding the mean difference of the values obtained by 2D and 3D TEE measures compared to the intra-operative standard, all values were significantly different, with the slightest difference observed in the SA 3D MPR images. The difference between echocardiographic and surgical measures could happen due to the dissimilar hemodynamic status during each of these methods. Since the intra-operative measurements were performed on a non-beating heart, echocardiographic studies were carry out under physiologic conditions.19 Studies utilizing intra-operative echocardiography can be beneficial in order to understand the source of this difference.23 However, further investigation is needed to support this hypothesis.
Limitations
In addition to the accuracy of three independent study methods compared to the surgical gold standard values, the prospective design of the present study is its other strength. However, this study also has some limitations. A major limitation of the present study is the small sample size. Although information related to different variables had been collected throughout this study’s course, the small sample size hampered the subgroup analysis in specific areas, such as the etiology of the disease and patients’ age.
Furthermore, because the researchers did not evaluate live (intraoperative) TEE, they could not establish whether the different MV annulus measures regarding the methods were related to the difference in patients’ hemodynamics and other conditions before and during the operation or it did primarily occur due to the imaging methods itself.
Study Highlights
What is current knowledge?
What is new here?
-
The present study highlights the differences in accuracy and reliability between 2D and 3D TEE measurements compared to the gold standard surgical ruler measurements. It suggests that 3D TEE, especially the 3D multiplanar reconstruction (MPR) method, shows a stronger correlation with intraoperative measures than 2D TEE.
Conclusion
This study showed a high correlation between 2D/3D TEE measured MV annulus and intraoperative using a surgical ruler as the gold standard method. Also, it has been concluded that the mitral annulus circumference in 3D–MPR TEE demonstrated the strongest correlation with intra-operative measures. Considering the lack of high quality studies on this subject, and the limitations of the present study, further studies should be performed for definite conclusions in this regard.
Competing Interests
The authors declare that they have no competing interests. Also, they should state that no financial supports and funding had been granted for the current study.
Ethical Approval
This study was approved by the Ethics Committee of Tabriz University of Medical Sciences (code: IR.TBZMED.REC.1399.589).
References
- Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M. Global epidemiology of valvular heart disease. Nat Rev Cardiol 2021; 18(12):853-64. doi: 10.1038/s41569-021-00570-z [Crossref] [ Google Scholar]
- Chen Y, Yiu KH. Growing importance of valvular heart disease in the elderly. J Thorac Dis 2016; 8(12):E1701-3. doi: 10.21037/jtd.2016.12.23 [Crossref] [ Google Scholar]
- Harb SC, Griffin BP. Mitral valve disease: a comprehensive review. Curr Cardiol Rep 2017; 19(8):73. doi: 10.1007/s11886-017-0883-5 [Crossref] [ Google Scholar]
- Guedes MA, Pomerantzeff PM, de Almeida Brandão CM, Vieira ML, Tarasoutchi F, da Cunha Spinola P. Mitral annulus morphologic and functional analysis using real time tridimensional echocardiography in patients submitted to unsupported mitral valve repair. Rev Bras Cir Cardiovasc 2015; 30(3):325-34. doi: 10.5935/1678-9741.20140082 [Crossref] [ Google Scholar]
- Cohn LH, Tchantchaleishvili V, Rajab TK. Evolution of the concept and practice of mitral valve repair. Ann Cardiothorac Surg 2015; 4(4):315-21. doi: 10.3978/j.issn.2225-319X.2015.04.09 [Crossref] [ Google Scholar]
- Cimino S, Guarracino F, Valenti V, Frati G, Sciarretta S, Miraldi F. Echocardiography and correction of mitral regurgitation: an unbreakable link. Cardiology 2020; 145(2):110-20. doi: 10.1159/000504248 [Crossref] [ Google Scholar]
- Castillo JG, Solís J, González-Pinto A, Adams DH. [Surgical echocardiography of the mitral valve]. Rev Esp Cardiol 2011; 64(12):1169-81. doi: 10.1016/j.recesp.2011.06.025.[Spanish] [Crossref] [ Google Scholar]
- Wunderlich NC, Beigel R, Ho SY, Nietlispach F, Cheng R, Agricola E. Imaging for mitral interventions: methods and efficacy. JACC Cardiovasc Imaging 2018; 11(6):872-901. doi: 10.1016/j.jcmg.2018.02.024 [Crossref] [ Google Scholar]
- Pastore MC, Mandoli GE, Sannino A, Dokollari A, Bisleri G, D’Ascenzi F. Two and three-dimensional echocardiography in primary mitral regurgitation: practical hints to optimize the surgical planning. Front Cardiovasc Med 2021; 8:706165. doi: 10.3389/fcvm.2021.706165 [Crossref] [ Google Scholar]
- Bartels K, Thiele RH, Phillips-Bute B, Glower DD, Swaminathan M, Kisslo J. Dynamic indices of mitral valve function using perioperative three-dimensional transesophageal echocardiography. J Cardiothorac Vasc Anesth 2014; 28(1):18-24. doi: 10.1053/j.jvca.2013.03.024 [Crossref] [ Google Scholar]
- Quader N, Rigolin VH. Two and three-dimensional echocardiography for pre-operative assessment of mitral valve regurgitation. Cardiovasc Ultrasound 2014; 12:42. doi: 10.1186/1476-7120-12-42 [Crossref] [ Google Scholar]
- Calleja A, Poulin F, Woo A, Meineri M, Jedrzkiewicz S, Vannan MA. Quantitative modeling of the mitral valve by three-dimensional transesophageal echocardiography in patients undergoing mitral valve repair: correlation with intraoperative surgical technique. J Am Soc Echocardiogr 2015; 28(9):1083-92. doi: 10.1016/j.echo.2015.04.019 [Crossref] [ Google Scholar]
- Poelaert JI, Bouchez S. Perioperative echocardiographic assessment of mitral valve regurgitation: a comprehensive review. Eur J Cardiothorac Surg 2016; 50(5):801-12. doi: 10.1093/ejcts/ezw196 [Crossref] [ Google Scholar]
- Fabricius AM, Walther T, Falk V, Mohr FW. Three-dimensional echocardiography for planning of mitral valve surgery: current applicability?. Ann Thorac Surg 2004; 78(2):575-8. doi: 10.1016/j.athoracsur.2003.10.031 [Crossref] [ Google Scholar]
- Coisne A, Pontana F, Aghezzaf S, Mouton S, Ridon H, Richardson M, et al. Utility of three-dimensional transesophageal echocardiography for mitral annular sizing in transcatheter mitral valve replacement procedures: a cardiac computed tomographic comparative study. J Am Soc Echocardiogr 2020;33(10):1245-52.e2. 10.1016/j.echo.2020.04.030.
- Tsang W, Lang RM. Three-dimensional echocardiography is essential for intraoperative assessment of mitral regurgitation. Circulation 2013; 128(6):643-52. doi: 10.1161/circulationaha.112.120501 [Crossref] [ Google Scholar]
- Ender J, Eibel S, Mukherjee C, Mathioudakis D, Borger MA, Jacobs S. Prediction of the annuloplasty ring size in patients undergoing mitral valve repair using real-time three-dimensional transoesophageal echocardiography. Eur J Echocardiogr 2011; 12(6):445-53. doi: 10.1093/ejechocard/jer042 [Crossref] [ Google Scholar]
- Ring L, Dutka DP, Wells FC, Fynn SP, Shapiro LM, Rana BS. Mechanisms of atrial mitral regurgitation: insights using 3D transoesophageal echo. Eur Heart J Cardiovasc Imaging 2014; 15(5):500-8. doi: 10.1093/ehjci/jet191 [Crossref] [ Google Scholar]
- Cong T, Gu J, Lee AP, Shang Z, Sun Y, Sun Q. Quantitative analysis of mitral valve morphology in atrial functional mitral regurgitation using real-time 3-dimensional echocardiography atrial functional mitral regurgitation. Cardiovasc Ultrasound 2018; 16(1):13. doi: 10.1186/s12947-018-0131-1 [Crossref] [ Google Scholar]
- Chen X, Li H, Feng Z, Tang S, Song L. Relationship between geometric changes in mitral annular/leaflets and mitral regurgitation in patients with atrial fibrillation. Medicine (Baltimore) 2019; 98(4):e14090. doi: 10.1097/md.0000000000014090 [Crossref] [ Google Scholar]
- Rajila Rajendran HS, Seshayyan S, Victor A, Murugesan N, Sundaramurthi I. The study of mitral valve annular dimension in relation to the body surface area in the Indian population. Eur J Cardiothorac Surg 2011; 39(5):653-6. doi: 10.1016/j.ejcts.2010.08.052 [Crossref] [ Google Scholar]
- Chen J, Li W, Xiang M. Burden of valvular heart disease, 1990-2017: results from the Global Burden of Disease Study 2017. J Glob Health 2020; 10(2):020404. doi: 10.7189/jogh.10.020404 [Crossref] [ Google Scholar]
- Sudhakar S, Khairnar P, Nanda NC. Live/real time three-dimensional transesophageal echocardiography. Echocardiography 2012; 29(1):103-11. doi: 10.1111/j.1540-8175.2011.01525.x [Crossref] [ Google Scholar]