J Res Clin Med. 12:33.
doi: 10.34172/jrcm.33376
Original Article
Radiological tips on safe tract selection in computed tomography-guided transthoracic biopsy: single-center results
Hanifi Koca Investigation, Resources, Writing – original draft, 1, * 
Mesut Özgökçe Conceptualization, Resources, Writing – review & editing, 2 
Muhammed Bilal Akıncı Visualization, 3 
Fatma Durmaz Formal analysis, Validation, 2 
Veysel Atilla Ayyıldız Project administration, 4 
Sercan Özkaçmaz Data curation, Software, 2 
Cemil Göya Methodology, Supervision, 2 
Author information:
1Department of Radiology, Şemdinli State Hospital, Hakkari, Türkiye
2Department of Radiology, Van Yuzuncu Yil University, School of Medicine, Van, Türkiye
3Department of Radiology, Gümüşhane State Hospital, Gümüşhane, Türkiye
4Department of Radiology, Süleyman Demirel Faculty of Medicine, Isparta, Türkiye
Abstract
Introduction:
The vast majority of lung masses are malignant. Benign lung masses include granulomatous inflammation and pneumonia consolidations. Malignant lung masses include lung cancers, lymphoma, and thymic neoplasms. Differentiating benign-malignant lung masses and treatment planning are essential for the prognosis of patients. Computed tomography (CT) guided transthoracic lung biopsy is a reliable diagnostic method with high accuracy and relatively few complications when an appropriate trace is selected. In this study, we aimed to present our experience and the results of lung mass cases that we biopsied with the guidance of CT.
Methods:
A total of 57 patients who were referred to us for clinicoradiological transthoracic biopsy (TTB) were studied with CT-guided histopathological sampling. The study did not include patients with no pathology results and ultrasound-guided biopsy.
Results:
A total of 57 patients, 42 male (73.6%) and 15 female (26.4%) with a mean age of 59.05±17.04 (1-85), were evaluated. Thirteen of the lesions were reported as benign (22.8%), and 44 as malignant (77.2%).
Conclusion:
When an appropriate trace is selected, CT-guided transthoracic lung biopsy is a reliable diagnostic method with high accuracy and relatively few complications.
Keywords: Computed tomography, Lung cancer, Lung masses, Transthoracic biopsy
Copyright and License Information
© 2024 The Authors.
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
There is no funding for the study.
Introduction
In recent years, lung cancer has been considered as one of the most common causes of death.1 Among all types of cancer, lung cancer is the first and the second most common cause of death in men and women, respectivley.2 Therefore, early diagnosis and treatment are very important. Clinical evaluation and radiological imaging are the main methods for early diagnosis and proper treatment. However, a histopathological diagnosis is required to make a precise differential diagnosis of the lesion, plan the medical and surgical treatments correctly, and prevent unnecessary thoracotomies.
Biopsies are divided into fine needle aspiration biopsy (FNAB) and tru-cut or cor biopsy. Thin-calibrated needles are used in FNAB, and aspiration material is taken for cytological evaluation. A tru-cut biopsy is known as a piece-by-piece biopsy and is based on the removal of a piece of tissue from the lesion for histological diagnosis.3,4
Computed tomography (CT)-guided transthoracic biopsy (TTB) is an effective method with high reliability and accuracy rates in the sampling and histopathological diagnosis of thoracic masses.4,5 In recent years, its usage has been increasing steadily and its accuracy rates are 80% and 90% in benign and malignant lesions, respectively.6 It is a relatively less invasive, inexpensive, and reliable method than invasive thoracotomy methods formerly used for histopathological diagnosis.
In this study, we aimed to present the appropriate tract selection techniques and histopathological results to improve the accuracy of diagnosis and reduce possible complications in CT-guided transthoracic biopsies in our clinic and present our histopathological results with the literature.
Methods
This study was approved by the Van Yuzuncu Yıl University Non-Invasive Clinical Research Ethics Committee. This study complied with the current Türkiye laws.
Patients
The data of patients, who underwent CT-guided lung mass biopsy in our interventional radiology clinic, were reviewed retrospectively between December 2018 and January 2019. Patients whose pathology results were insufficient and whose radiological or pathological data could not be reached, were excluded from the study. The study was conducted with 57 patients who underwent CT-guided biopsy.
Age and gender of the patients; location, size, and features of lesions on CT images, skin-pleura and pleura-lesion distance, the relationship between lesion and fissure, and the presence of emphysema were evaluated.
Biopsy Procedure
The coagulation parameters of all patients were checked before the biopsy procedure. Detailed information about the biopsy technique and the complications was given to the patients/patient relatives and written consent were obtained. The biopsy was performed using a 16-slice and 64-slice (Siemens Medical Systems, Erlangen, Germany) multislice CT device (dose parameters 140 mAs, 120 kV, slice thickness 2.5 mm, reconstruction interval 3 mm, FOV 320 mm (axial plane)). In lesions that were located close to the thoracic wall and clearly visualized by the US, a biopsy was performed by a Philips brand Ultrasound device (4 C convex probe).
Biopsy was not performed in some patients who recovered in control CT scans. As a result of the council’s decisions, a biopsy decision was made for all of our cases. The multidisciplinary approach reduced unnecessary invasive procedure rates by making a biopsy decision according to the lesions’ radiological, clinical, and follow-up conditions. The biopsy was performed using an 18 G tru-cut biopsy device with a coaxial system.
The patient’s hospitalization position was determined as supine, prone, or lateral decubitus by evaluating the previous CT images and planning the entry distance from the closest location to the lesion. If a lesion is on the anterior, the supine position; if it is on the posterior, the prone position, and if it is located close to the lateral thoracic wall, the lateral decubitus position is selected. By looking at the previous CT images of the patient, the patient was hospitalized in the most appropriate place planned for the intervention. Then, an axial CT scanogram scan including the lesion was performed. After that, a metallic marker (closed injector tip) was placed on the skin and the image was retaken (Figure 1).
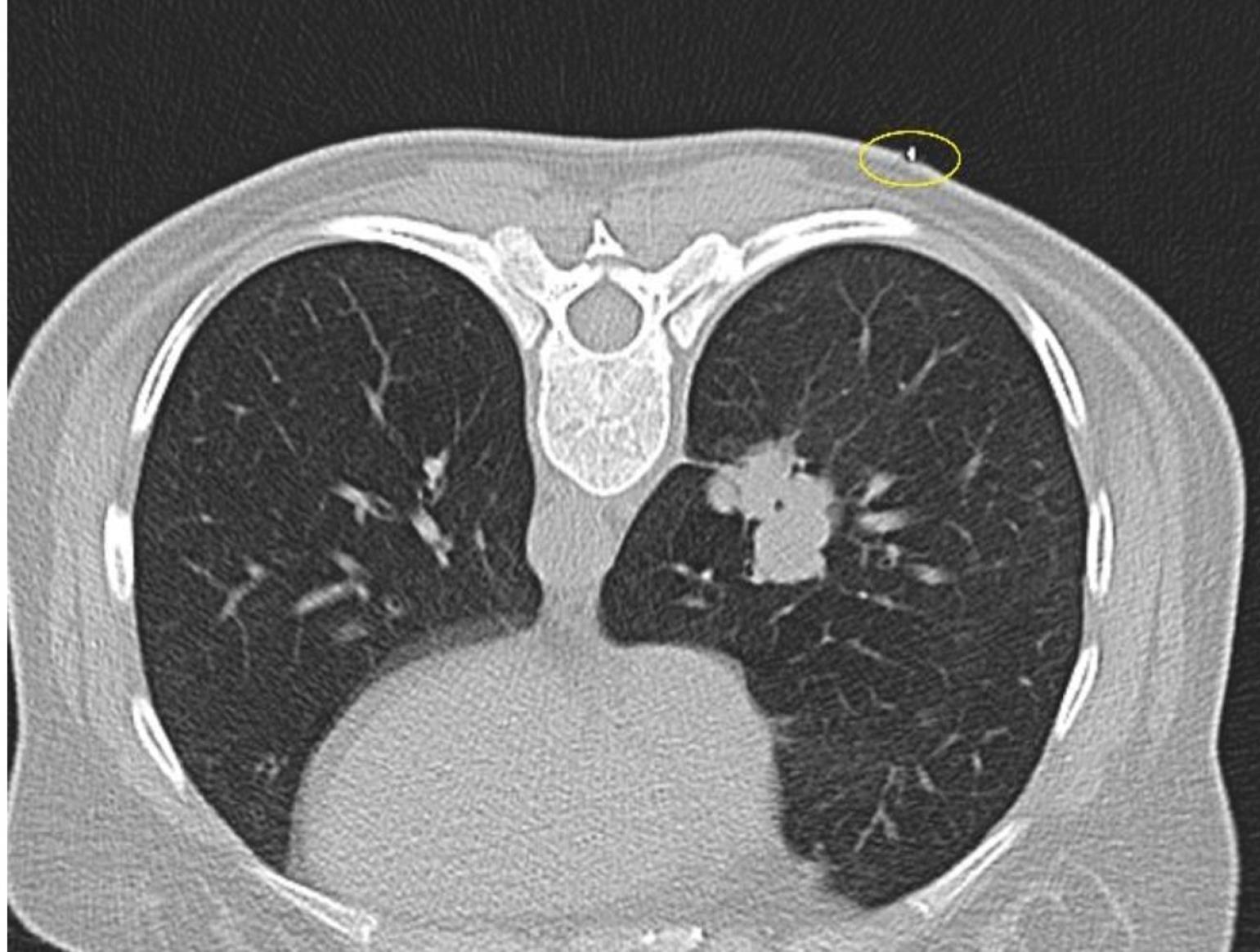
Figure 1.
The marker is used for localization on the skin (yellow circle) in the right lung mass lesion CT from the most suitable prone position
.
The marker is used for localization on the skin (yellow circle) in the right lung mass lesion CT from the most suitable prone position
The location and distance of entry to the lesion have been rescheduled according to this image. The skin-pleura and pleura-lesion distances were calculated (Figure 2). Care was taken to ensure that there are no areas such as fissures, bullae, major vascular structures, ribs, and scapula that prevent entry in the entry tracing. The process was continued either by changing the gantry angle or finding another safe way. After the entry point, angle and trace were determined and the area was cleaned with an antiseptic solution and covered with a perforated sterile drape. Local anesthesia was administered to the entry point under sterile conditions. 5-10 cc prilocaine HCL (Citanest®) was used for local anesthesia.
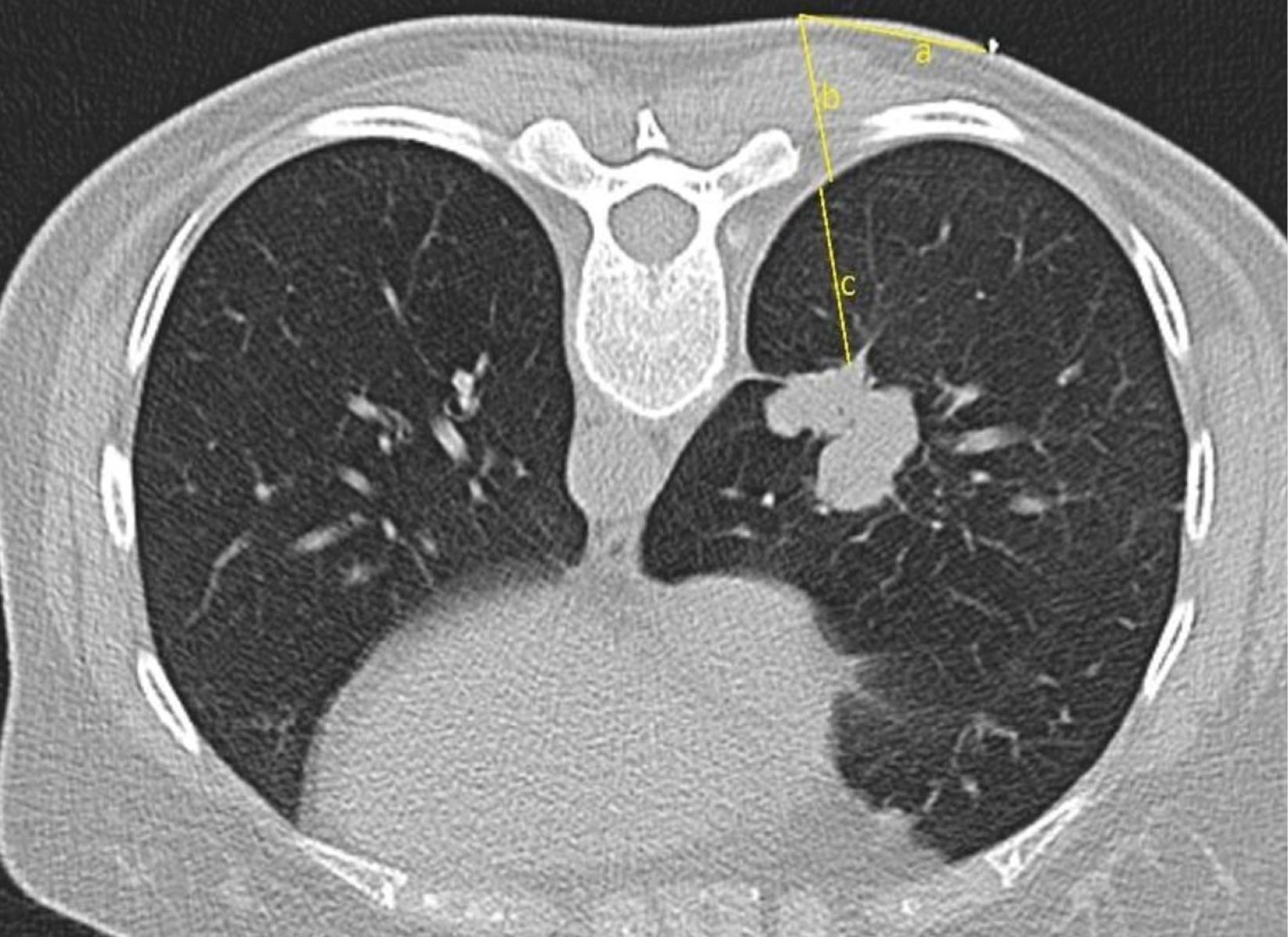
Figure 2.
Measurement of the distances from the skin to the most appropriate angle (a), from the possible entry point to the pleura (b), and from the pleura to the mass (c)
.
Measurement of the distances from the skin to the most appropriate angle (a), from the possible entry point to the pleura (b), and from the pleura to the mass (c)
With a sterile Chiba needle tip, the skin and subcutaneous tissue were passed a little, the lesion area was re-imaged, and the needle-lesion tract was controlled and planned. During passing the pleura; the lesion was quickly entered at a time as much as the angle and distance calculated earlier. The image was retaken to check that the needle was in the appropriate place (Figure 3). All tissue samples were obtained with a tru-cut device (18 Gauge, 17 cm) several times through a Chiba needle (17 Gauge, 15 cm) called a coaxial system. After making sure that the needle tip was in the lesion, the biopsy was performed with a tru-cut device until the desired amount of tissue was obtained in the Chiba needle (approximately two or sometimes three times on average).
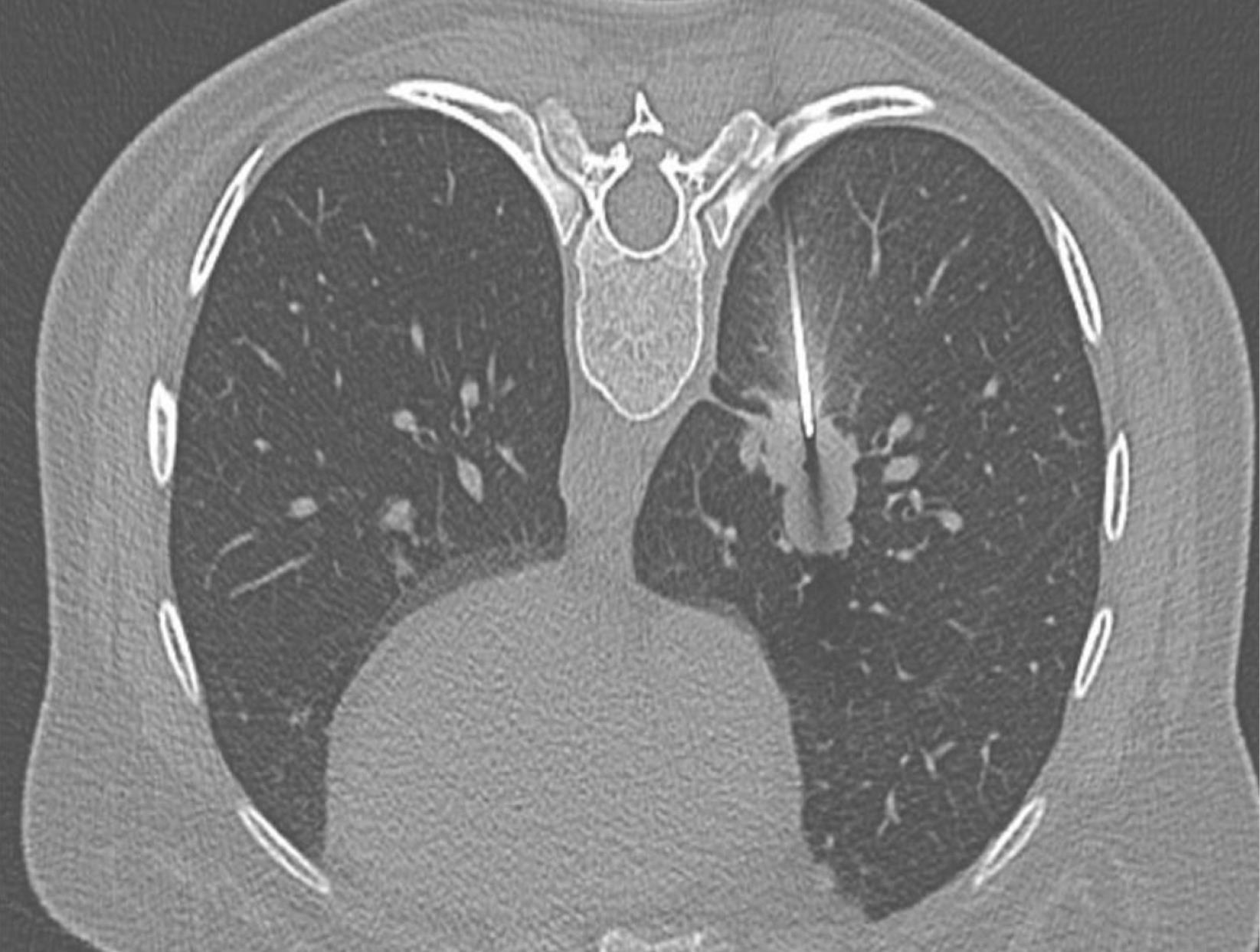
Figure 3.
View of Chiba needle in the mass
.
View of Chiba needle in the mass
The biopsy piece taken at least twice was transferred into a container containing 10% formalin. After the biopsy procedure, the entry site was covered with gauze and plaster. The final control imaging was taken and evaluated for complications such as pneumothorax and parenchymal hemorrhage. Then, patients who did not develop serious complications were sent to the ward on a stretcher to be followed up for at least 2 hours so that the needle location remained at the bottom. Patients whose pathology was not detected on the control lung X-ray and those who did not have any complaints clinically were discharged. The cases that developed pneumothorax were followed up for 4 hours at 2-hour intervals, or they were hospitalized in the thoracic surgery service for a chest tube and kept under observation.
Statistical analysis
Descriptive statistics for continuous variables in our study are expressed as mean, standard deviation, minimum and maximum values. In the study, descriptive statistics for continuous variables are expressed as mean, standard deviation, minimum, and maximum values; while categorical variables are expressed as numbers and percentages. SPSS (version 23) statistical package program was used for calculations where the statistical significance level was taken as 5%.
Results
A total of 57 patients, 42 male (73.6%) and 15 female (26.4%) with a mean age of 59.05 ± 17.04 (1-85), were evaluated (Table 1). The mean ages of the benign and malignant patients were 59.00 ± 15.81 (22-81) and 59.06 ± 17.56 (1-85), respectively. Thirteen of the lesions were reported as benign (22.8%) and 44 as malignant (77.2%) (Table 2). Patients with malignant results was detected as 38.59% (22 patients) adenocarcinoma), 10.52% (six patients) squamous cell carcinoma, 5.2% (three patients) metastasis, 8.7% (five patients) small cell carcinoma, 3.5% (two patients) non-small cell tumor, and 10.52% (six patients) lymphoma, multiple myeloma, Ewing’s sarcoma, neuroblastoma, histiocytic sarcoma, and plasma cell myeloma (Figure 4).
Table 1.
Percentage of genders
|
Male
|
Female
|
Total
|
|
n
|
%
|
n
|
%
|
n
|
%
|
| 42 |
73.6 |
15 |
26.4 |
57 |
100 |
Table 2.
Percentage of benign and malignant patients and mean age
|
|
n
|
%
|
Mean age
|
| Benign |
13 |
22.8 |
59.00 |
| Malignant |
44 |
77.2 |
59.06 |
| Total |
57 |
100 |
59.05 |

Figure 4.
Malign lung lesions
.
Malign lung lesions
The mean size of the lesions was 59 mm, the skin-pleural distance was 32 mm, and the pleura-lesion space was 11 mm (Table 3). Twelve lesions were located in the right upper lobe, 5 in the right middle lobe, 12 in the right lower lobe, 16 in the left upper lobe, 8 in the left lower lobe, and 4 in the mediastinum (Figure 5).
Table 3.
Skin-pleura and pleura-lesion distance
|
|
All patient
|
Pneumothorax
|
| Mean skin-pleura distance (mm) |
32 |
33,12 |
| Mean pleura-lesion distance (mm) |
11 |
19,25 |
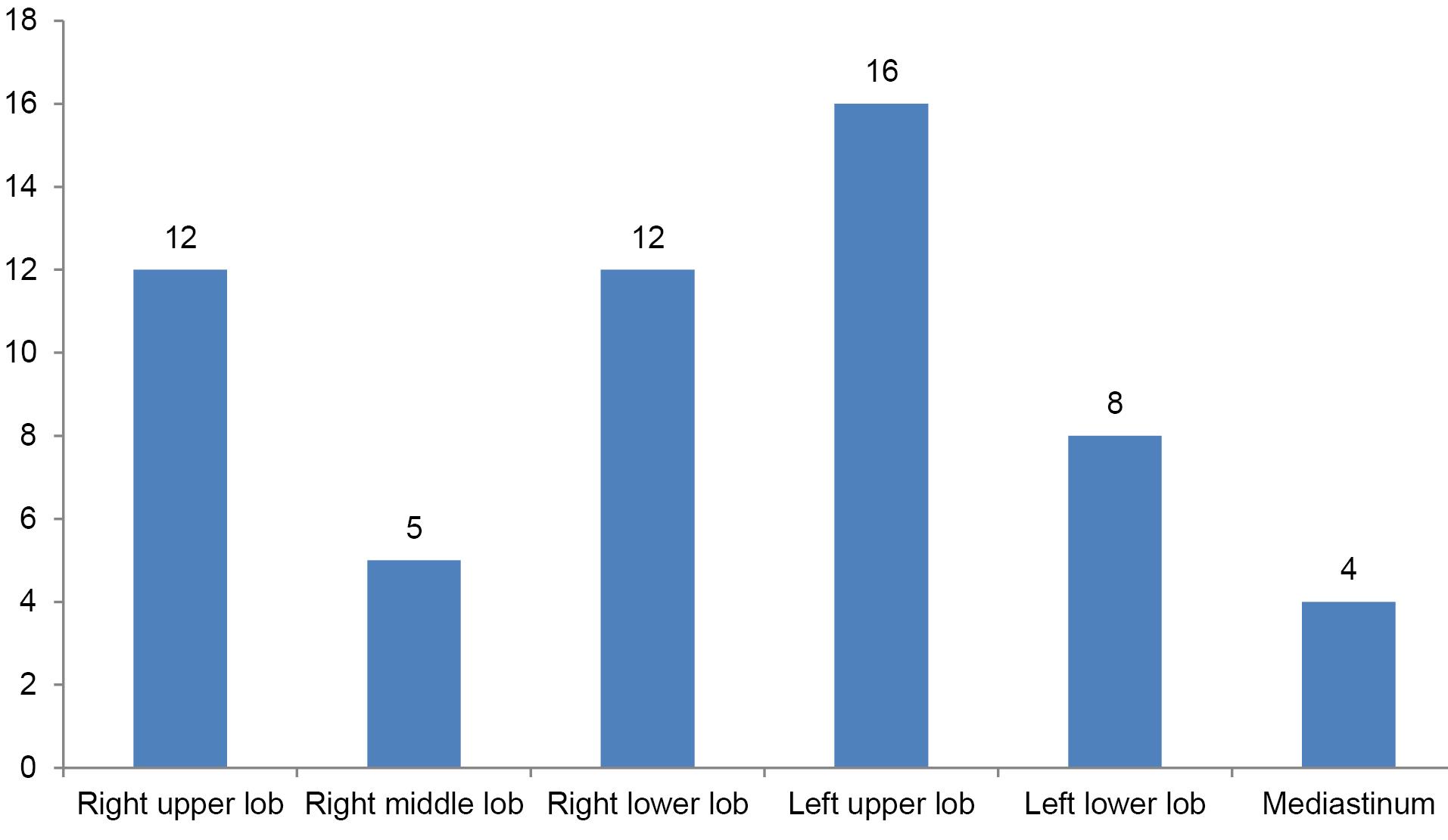
Figure 5.
Distribution of lesions to lung lobes
.
Distribution of lesions to lung lobes
Mild pneumothorax was observed in eight patients’ biopsies. Chest tube insertion was required in a patient. All pneumothorax complications occurred during the procedure and the other seven cases regressed in the follow-up. The mean skin-pleura distance was 33.12 mm, and the mean pleura-lesion distance was 19.25 mm in patients who developed pneumothorax (Table 3).
Mild hemorrhagic contusion was observed in the tract localization in three of patients. No fissure or bulla-blep was passed during the procedure in any patient. Air embolism and hemoptysis occurred in one of patients.
Discussion
CT-guided TTB indications include solid or multiple pulmonary nodules, mass lesions, persistent focal infiltrations, cavitary lesions, and mediastinal, hilar, and pleural masses and lesions.5
Pneumothorax is the most common complication in patients undergoing CT-guided TTB with a rate of 8%-64% reported in the literature.7-10 In the vast majority of cases, pneumothorax regresses by itself, and in 2%-31%, it is necessary to use a chest tube. Obstructive lung disease, emphysema, the size of the target lesion, the number of biopsies, the passage of the fissure, the size of the biopsy needle, age, and operator experience are the factors that affect the risk of pneumothorax.11 Therefore, all these features should be reevaluated for each patient with a CT scan. Pneumothorax complications developed in eight of our patients (14%). Most of them were old patients. This risk is higher in elderly patients whose parenchyma cannot be evaluated well. Spontaneous regression occurred in seven of our pneumothorax cases, except for one.
The distance of the lesion to the pleura is another factor that increases the risk of pneumothorax.11 In our study, we found that increasing both the pleura-lesion and the skin-pleural distance increased the risk of pneumothorax.
The second most common complication is pulmonary hemorrhage. Studies have reported that it develops in 15%-26% of patients. It has been reported that it is associated with hemoptysis at a rate of 4-5%.12 The distance of the lesion to the pleura, the cavitation of the lesion, the presence of bronchiectasis, and the diameter of the biopsy needle are associated with pulmonary hemorrhage.5,13 Mild parenchymal contusions occurred in three patients, and minimal hemoptysis occurred in one of them, however, whose complaint was disappeared completely within 2 hours.
Since lung cancer is the most common cause of death worldwide, the main goal in lung masses is to differentiate malignant benign with appropriate radiological and bronchoscopic modalities. The vast majority of lung masses are malignant.14 86.27% of our cases were reported as malignant. Benign lung masses include pneumonic consolidations and granulomatous inflammation. Malignant lung masses include lung cancers, lymphoma, and thymic neoplasms. In our study, 35 (61.4%) patients had primary lung cancer. The histopathology results of lung masses in our region were found close to the literature.
The low number of cases in our study is a limitation. However, we think that the main reason is the exclusion of US-guided pleural-based biopsies and results in private pathology centers. Another limitation is that it is a retrospective study. However, we already aimed to share our experiences with biopsy results and complications.
Study Highlights
What is current knowledge?
CT guided transthoracic lung biopsy is a reliable diagnostic method with high accuracy and relatively few complications when an appropriate trace is selected. CT-guided TTB is an effective method with high reliability and accuracy rates in the sampling and histopathological diagnosis of thoracic masses.
What is new here?
Determining the appropriate tracing in experienced hands is very important for a low complication rate.
Conclusion
The CT-guided transthoracic biopsy procedure is easy to perform, has low cost and high diagnostic accuracy, and is safe with a severely low complication rate. Examining the tomography images in advance can allow choosing the most suitable and safest track with experienced hands.
Acknowledgements
This article is resulted from a study registered at Van Yuzuncu Yil University.
Competing Interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical Approval
This study was approved by the Van Yüzüncü Yıl University Non-Invasive Clinical Research Ethics Committee (Date: 10.09.2021, decision no: 2021/10-11). The study has been conducted in accordance with the international declaration, guidelines, etc.
References
- Schabath MB, Cote ML. Cancer progress and priorities: lung cancer. Cancer Epidemiol Biomarkers Prev 2019; 28(10):1563-79. doi: 10.1158/1055-9965.Epi-19-0221 [Crossref] [ Google Scholar]
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011; 32(4):605-44. doi: 10.1016/j.ccm.2011.09.001 [Crossref] [ Google Scholar]
- Verma R, Sood S, Sarma S, SharmaN SS, Sarkar M. Single puncture CT guided fine-needle aspiration cytology (FNAC) and tru-cut biopsy in indeterminate lung lesions. J Med Sci Clin Res 2017; 5(7):24796-802. doi: 10.18535/jmscr/v5i7.83 [Crossref] [ Google Scholar]
- Manhire A, Charig M, Clelland C, Gleeson F, Miller R, Moss H. Guidelines for radiologically guided lung biopsy. Thorax 2003; 58(11):920-36. doi: 10.1136/thorax.58.11.920 [Crossref] [ Google Scholar]
- Yılmaz A, Akkaya E, Baran R. Transtorasik iğne aspirasyonu. Tüberküloz ve Toraks 2002; 50(2):295-300. [ Google Scholar]
- Beşir FH, Altın R, Kart L, Akkoyunlu M, Ozdemir H, Ornek T. The results of computed tomography guided tru-cut transthoracic biopsy: complications and related risk factors. Wien Klin Wochenschr 2011; 123(3-4):79-82. doi: 10.1007/s00508-011-1538-y [Crossref] [ Google Scholar]
- Poulou LS, Tsagouli P, Ziakas PD, Politi D, Trigidou R, Thanos L. Computed tomography-guided needle aspiration and biopsy of pulmonary lesions: a single-center experience in 1000 patients. Acta Radiol 2013; 54(6):640-5. doi: 10.1177/0284185113481595 [Crossref] [ Google Scholar]
- Beslic S, Zukic F, Milisic S. Percutaneous transthoracic CT guided biopsies of lung lesions; fine needle aspiration biopsy versus core biopsy. Radiol Oncol 2012; 46(1):19-22. doi: 10.2478/v10019-012-0004-4 [Crossref] [ Google Scholar]
- Loh SE, Wu DD, Venkatesh SK, Ong CK, Liu E, Seto KY. CT-guided thoracic biopsy: evaluating diagnostic yield and complications. Ann Acad Med Singap 2013; 42(6):285-90. [ Google Scholar]
- Tsukada H, Satou T, Iwashima A, Souma T. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. AJR Am J Roentgenol 2000; 175(1):239-43. doi: 10.2214/ajr.175.1.1750239 [Crossref] [ Google Scholar]
- Li Y, Du Y, Yang HF, Yu JH, Xu XX. CT-guided percutaneous core needle biopsy for small ( ≤ 20 mm) pulmonary lesions. Clin Radiol 2013; 68(1):e43-8. doi: 10.1016/j.crad.2012.09.008 [Crossref] [ Google Scholar]
- Zhu J, Qu Y, Wang X, Jiang C, Mo J, Xi J. Risk factors associated with pulmonary hemorrhage and hemoptysis following percutaneous CT-guided transthoracic lung core needle biopsy: a retrospective study of 1,090 cases. Quant Imaging Med Surg 2020; 10(5):1008-20. doi: 10.21037/qims-19-1024 [Crossref] [ Google Scholar]
- Rizzo S, Preda L, Raimondi S, Meroni S, Belmonte M, Monfardini L. Risk factors for complications of CT-guided lung biopsies. Radiol Med 2011; 116(4):548-63. doi: 10.1007/s11547-011-0619-9 [Crossref] [ Google Scholar]
- Jiang J, Fu Y, Hu X, Cui L, Hong Q, Gu X. The value of diffusion-weighted imaging based on monoexponential and biexponential models for the diagnosis of benign and malignant lung nodules and masses. Br J Radiol 2020; 93(1110):20190400. doi: 10.1259/bjr.20190400 [Crossref] [ Google Scholar]