J Res Clin Med. 12:1.
doi: 10.34172/jrcm.2024.33310
Original Article
The effect of luteal phase prolongation with medroxyprogesterone acetate on endometrial thickness and pregnancy rate in women following recurrent implantation failure
Behnaz Sadeghzadeh Oskouei Conceptualization, Funding acquisition, Investigation, Project administration, Visualization, Writing – review & editing, 1 
Parviz Shahabi Resources, Validation, Writing – review & editing, 1
Hossein Babaei Software, Supervision, Writing – original draft, 1
Azizeh Farshbaf-Khalili Data curation, Formal analysis, Methodology, Writing – original draft, 2, * 
Author information:
1Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Physical Medicine and Rehabilitation Research Centre, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Assisted reproductive technology (ART) is increasingly used to help infertile couples to have a child. However, less than one-third of transferred embryos result in successful implantation and live birth. Recurrent implantation failure (RIF) is an outstanding barrier to pregnancy achievement by this advanced technology. This study was conducted to evaluate the hypothesis that luteal phase prolongation would lead to endometrial thickening and decrease the RIF’s rate.
Methods:
This interventional study was conducted in the infertility clinic of the Alzahra Teaching Hospital and Asadabadi polyclinic, Tabriz, Iran. Sixty women, aged 20–40 years, with a history of RIF following several in vitro fertilization treatment cycles with mid-luteal progesterone levels less than 3 ng/mL, and endometrial thickness less than 8mm were recruited through the purposive sampling method. Participants were treated for six cycles with oral medroxyprogesterone acetate (MPA) tablet 5 mg/12 h initiated from the 16th day of their menstrual cycle for 20 days and evaluated for study outcomes during this course. Endometrial thickness was compared before and after MPA administration in the whole study population. The pregnancy rate was determined. Pregnancy complications and conditions were evaluated during the study.
Results:
Endometrial thickness was increased in patients after MPA administration (mean difference [95% CI]: 2.66 [2.43 to 2.89]; P<0.001). Overall, 80% (n=48) of participants achieved biochemical pregnancy during a 6-month treatment. Four pregnancies out of 48, were lost in different stages of pregnancy. One participant experienced gestational diabetes in the 24th week of pregnancy. No embryo-fetal abnormality was present until delivery.
Conclusion:
Administration of MPA following RIF induces endometrial development and facilitates embryo implantation by luteal phase prolongation which results in natural pregnancy achievement followed by timed intercourse.
Keywords: Endometrial thickness, Luteal phase, Recurrent implantation failure
Copyright and License Information
© 2024 The Authors.
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study resulted from a research proposal that has been approved and financially supported by the Drug Applied Research Center, Tabriz University of Medical Sciences (Grant no: 57541).
Introduction
Infertility affects about 80 million couples worldwide. Assisted reproductive technology (ART), plays a critical role during in vitro production of human infertility treatments.1 However, ART procedures consist of several steps. It is so expensive and it has extensive side effects. Therefore it should be used only if necessary.2
ART is increasingly used to help infertile couples to have a child. However, less than one-third of transferred embryos result in successful implantation and live birth. Recurrent implantation failure (RIF) is an outstanding barrier to pregnancy achievement by this advanced technology. The term RIF has been used since 1983 and is defined as “pregnancy failure following 2–6 consecutive embryo transfer cycles, in which more than 10 good quality embryos were transferred to the uterus”.3-5 Approximately 10% of women undergoing ART treatments interface to RIF. Over the last decades, RIF has been introduced as a critical clinical impediment of ART.6-8 One of the underlying pathophysiological mechanisms of RIF may be the post-ovulatory production of excessive steroids and the abnormal hormonal milieu and may therefore potentially interfere with the luteal activity of normal ovulatory women9,10 due to inessential ovarian stimulation during ART cycles. This hormonal milieu is detrimental to endometrial receptivity for embryo implantation.
The endometrium is a fundamental portion of ART success; as a matter of fact, endometrial thickness has been proposed as a predictor of implantation.11,12 As a result, couples with unexplained infertility and/or RIF are disappointingly stranded in a reproductive vicious cycle.
The measure of endometrial thickness has been proven to be effective in pregnancy outcomes. The thin endometrium is generally defined as < 7 mm on the day of ovulation.13 A decreased endometrial thickness has been reported to be associated with embryo implantation failure.14 In ART cycles, the incidence of thin endometrium has been reported to be 1.5%-9.1%. Such differences in incidence could be related to kinds of measurement techniques; such as ultrasound equipment, ART protocols, etc.13 In reproductively normal couples, a decreased endometrial thickness indicates an increased risk of infertility and/or pregnancy loss.14 These pieces of evidence render the urgent need for novel treatments for this problem.
The current interventional study is an investigation of a new treatment for women with RIF. This study aimed to evaluate the effect of luteal phase prolongation with medroxyprogesterone acetate (MPA) on endometrial thickness and pregnancy rate in women with RIF through natural intercourse. To our knowledge, the present method has not been reported in previous studies about ART. It is worth mentioning here that the present technique is not an adaptation or extension, it is a distinct method in its own right.
Methods
Design and setting of the study
We conducted a before and after interventional study at the infertility clinic of the Alzahra teaching hospital and Asadabadi polyclinic, Tabriz, Iran. Participants were enrolled among women aged 20-40 years with RIF following several in vitro fertilization treatment cycles six months before the study, mid-luteal progesterone levels less than 3 ng/mL on the 21st day of the menstrual cycle, and mid-luteal endometrial thickness less than 8 mm in abdominal ultrasonography, had a university education, and consented to participate in a quasi-experimental study on the efficacy of prolongation of luteal phase as a treatment of RIF. Women aged older than 40 years, individuals with abnormal semen analysis of husbands, previous ovarian surgery, oophorectomy, hypogonadotropic hypogonadism, and diminished ovarian reserve were excluded.
Sampling and intervention
Sixty eligible women with thin endometrium and mid-luteal progesterone levels less than 3 ng/ml on the 21st day of the menstrual cycle were recruited through the purposive sampling method. All participants for six cycles received oral MPA (Tablet Medrofem 5 mg; Iran Hormone, Tehran, Iran) with a dosage of 5 mg/12 h initiated from the 16th day of their menstrual cycle for 20 days and evaluated for study outcomes during this course.
No other induction ovulation agents such as clomiphene citrate, or human menopausal gonadotropin (hMG) were prescribed. The study participants were recommended to have sexual intercourse from the 10th day of the menstrual cycle every other day for 10 times (possible ovulation days).
The data collection tool was a questionnaire that included demographic and obstetric characteristics consisting age, job, education, marriage duration, history of intrauterine fetal death (IUFD), type of ART, ART rate, gravida, para, abortion, and a sheet for recording the baseline and after intervention endometrial thickness (mm) and serum hormonal levels. The validity of these tools was confirmed by eight academic members with related expertise.
Serum β-hCG (human chorionic gonadotropin), progesterone, and estradiol levels were measured before and after intervention with MPA using the enzyme-linked immunosorbent assay (ELISA) method by the same kits and a specific laboratory expert. Ultrasonic assessment (A real-time ultrasonic device, Aloka 3500, Hitachi Aloka, America) of endometrial thickness was carried out before and after the termination of MPA treatment by the same device and radiologist. Clinical pregnancy was evaluated in the second week of pregnancy after a positive β-hCG test by ultrasound. Also, an ultrasound assessment was carried out in the 7th week for verification of a healthy pregnancy by visible heart rate. In cases of pregnancy achievement, standard obstetric care and follow-up were performed until birth.
Thin endometrium was considered as mid-luteal endometrial thickness less than 8mm in abdominal ultrasonography.
Statistical analysis
Statistical analyzes were performed using SPSS 22 software for Windows (Chicago, IL, USA). The normality of data was assessed through Kolmogorov-Smirnov test. Results were expressed as mean ± SD and percentages. Descriptive analysis was performed and the paired-samples t-test was applied for intra-group comparison. A statistically significant difference was considered at a P value < 0.05.
Results
A total of 60 women were included in the study from February 2017 to March 2019 following the assessment of eligibility criteria (Figure 1).
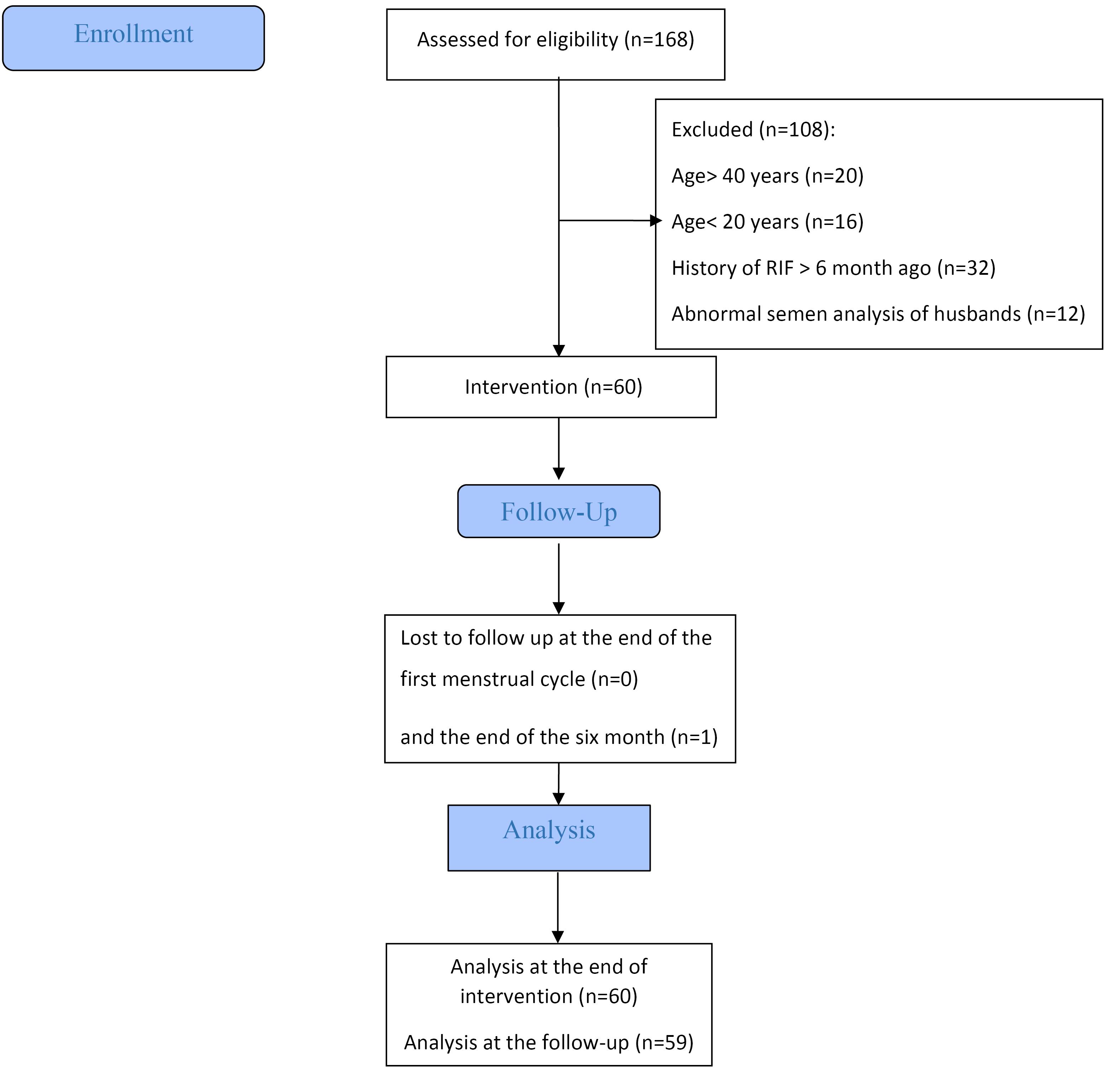
Figure 1.
Flowchart of study
.
Flowchart of study
Socio-individual characteristics of the participants are presented in Table 1. In the present study, the average age of participants was 28.6 years (range, 21–37). The mean (SD) infertility duration was 4.1 (1.7) years. Nearly 60% of them had a history of one to three failed ART.
Table 1.
Socio-individual characteristics of study women
|
Variable
|
n (%)
|
| Age (year)/ mean (SD) |
28.6 (4.9) |
| 21-25 |
25 (41.7%) |
| 26-30 |
11 (18.3%) |
| 31-37 |
24 (40.0%) |
| Job |
|
| Employed |
26 (43.3%) |
| Unemployed |
34 (56.7%) |
| Education |
|
| BSc |
36 (60.0%) |
| MSc |
18 (30.0%) |
| PhD |
6 (10.0%) |
| Marriage duration (year) |
5.0 (2.3) |
| Infertility duration (year) |
4.1 (1.7) |
| IUFD |
4 (6.7%) |
| Type of ART |
|
| IUI |
6 (10.0%) |
| ICSI |
54 (90.0%) |
| Gravida/ mean (SD) |
4.4 (1.9) |
| 3 |
14 (13.3%) |
| 4 |
22 (36.7%) |
| 5 |
14 (23.4%) |
| 6-7 |
10 (16.6%) |
| Para |
|
| 0 |
56 (93.3%) |
| 1 |
3 (5%) |
| 2 |
1 (1.7%) |
| Abortion/ mean (SD) |
4.3 (1.0) |
| 3 |
14 (23.3%) |
| 4 |
24 (40.0%) |
| 5 |
14 (23.3%) |
| 6-7 |
8 (13.4%) |
| ART rate/mean (SD) |
6.6 (2.5) |
| 1-3 |
35 (58.4%) |
| 4-6 |
25 (41.6%) |
IUFD, intrauterine fetal death; ART, Assisted reproductive technology; IUI, Intrauterine insemination; ICSI, Intracytoplasmic sperm injection.
Administration of MPA significantly increased mean endometrial thickness from 5.80 ± 0.91 mm at baseline to 8.47 ± 1.09 mm (mean difference [95% CI]: 2.66 [2.43 to 2.89] mm; P < 0.001) (Table 2). Forty-eight out of the 60 participants (80%) achieved biochemical pregnancies during a 6-month treatment. Clinical pregnancy was confirmed as an observable gestational sac in the uterine at the second week of pregnancy after a positive β-hCG test by ultrasound. Serum progesterone increased significantly after intervention (mean difference [95% CI]: 7.37 [6.88 to 7.87]; P < 0.001). Change in estradiol level was not significant (mean difference [95% CI]: 0.21 [-0.29 to 0.71], P = 0.399) (Table 3).
Table 2.
Results of descriptive statistics and paired sample t-test for endometrial thickness before and after MPA administration
|
Outcome
|
Before MPA (n=60)
|
After MPA (n=60)
|
Mean difference (95% CI)
|
P*
|
|
Mean
|
SD
|
Mean
|
SD
|
| Endometrial thickness (mm) |
5.80 |
0.91 |
8.47 |
1.09 |
2.66 (2.43 to 2.89) |
< 0.001 |
Table 3.
Results of serum progesterone and estradiol level in participants
|
Outcome
|
Before MPA (n=60)
|
After MPA (n=60)
|
Mean difference (95% CI)
|
P*
|
|
Mean
|
SD
|
Mean
|
SD
|
| Progesterone level (ng/mL) |
4.63 |
0.94 |
12.01 |
1.97 |
7.37 (6.88 to 7.87) |
< 0.001 |
| Estradiol level (pg/mL) |
54.60 |
7.64 |
54.81 |
7.31 |
0.21 (-0.29 to 0.71) |
0.399 |
Next, an ultrasound assessment was carried out in the 7th week to verify a healthy pregnancy by visible heart rate. One pregnancy was lost due to arrested embryonic development in the 8th week of pregnancy. One pregnancy was lost because of ectopic pregnancy. Another one was lost due to IUFD and the last one, was subjected to amniocentesis because of an abnormal screening test, she carried an embryo with Turner syndrome. Shared decision-making was made and the embryo was maintained; however premature rupture of membrane (PROM) at the 25th week resulted in preterm labor and the fetus do not stay alive. 44 pregnancies resulted in live healthy births. One participant experienced gestational diabetes in the 24th week of pregnancy. No embryo abnormality was present until delivery. Notably, no participant experienced preeclampsia, or eclampsia during pregnancy and no embryo-fetal abnormalities were reported during pregnancy care. Ultimately 15 out of the remaining pregnancies resulted in live healthy births by natural vaginal delivery (NVD), and 29 by elective cesarean section (CS) (Figure 2).
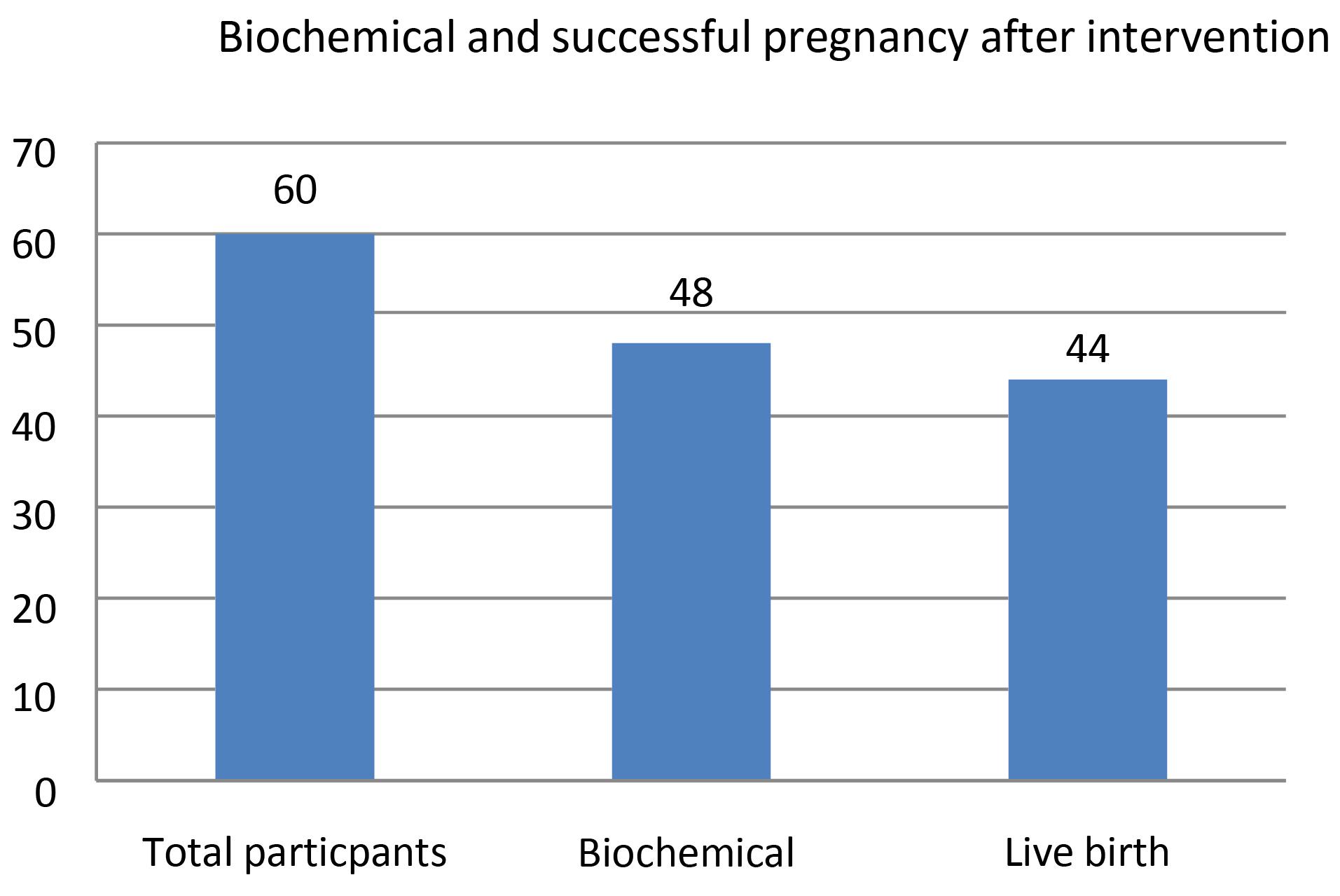
Figure 2.
Frequency of biochemical and successful pregnancy after intervention among total participants
.
Frequency of biochemical and successful pregnancy after intervention among total participants
Discussion
This study, for the first time, demonstrates that an oral mono-drug medication for successful treatment after RIF results in pregnancy achievement by natural intercourse in most of the affected cases. The present protocol does not need any induction ovulation agents. The key to success in this protocol is the correct choice of patients.
Implantation occurs in the luteal phase. Successful implantation depends on progesterone. This agent converts endometrium from a proliferative state to a secretory state and inhibits spontaneous uterine contractions.15
Progesterone is a steroid hormone that plays an essential role in the reproductive process. It plays a role in the menstrual cycle and implantation and is very essential for every stage of human pregnancy. The physiological impacts of progesterone are mainly mediated by interaction with the progesterone receptor.16 Progesterone causes secretory changes in the mucous membrane of the uterus and is necessary for the successful implantation of the embryo. In early pregnancy, progesterone is secreted by the corpus luteum, whose lifespan is estimated to be 12 ± 2 days. It is essential to maintain the pregnancy until the placenta (syncytiotrophoblast) assumes its function at 7-9 weeks of pregnancy. Therefore, progesterone supplementation can reduce the risk of miscarriage in women with a history of recurrent miscarriages.17 In addition, progesterone regulates the mother’s immune response. It promotes uterine relaxation and suppresses uterine contractions to prevent fetal rejection.16
MPA, a 17α-hydroxyprogesterone derivative, is a synthetic analog of the natural steroid progesterone. MPA has a more favorable bioavailability and a longer half-life than progesterone.18
The idea of using MPA as a treatment for RIF emerged from careful attention to the menstrual cycle physiology. Naturally, the endometrial preparation for embryo implantation begins at the proliferative phase and extends throughout the luteal phase. It has been demonstrated that the luteal phase is deficient in some cases of RIF and undergoing several ART cycles. Although several causes for this deficiency have been suggested, however, the substantial mechanism remains unknown.9,10 The use of gonadotropin-releasing hormone (GnRH) agonists in ART cycles, for controlled ovarian stimulation, produces an enhanced inhibition of the hypothalamus-pituitary-gonadal axis resulting from more steroids secreted by multiple corpora lutea. Consequently, luteinizing hormone (LH) levels are lowered due to high levels of steroids.19,20 It has been suggested that supraphysiologic serum steroid concentrations might adversely affect LH secretion via feedback mechanisms, which in turn results in premature luteolysis, defective progesterone secretion, and finally luteal phase defect (LPD).8 Inadequate secretion of progesterone can cause miscarriage in early pregnancy11,12 and possibly, result in unexplained RIF due to endometrial weakness.12 In ART cycles, progesterone or hCG levels, or both, are insufficient, therefore the luteal phase needs to be supported. Nevertheless, the optimal method for luteal phase supplementation is unclear7 and remains a matter of debate.21 Thus far, in order to improve pregnancy outcomes in unexplained RIF patients, researchers have applied various methods such as mechanical endometrial injury,2 ART, or high-dose vaginal progesterone gel/suppository. However, these methods are invasive or inconvenient and success rates are low.7
Despite recent progress in ART, the implantation process has a relatively low success rate.6,22 This could be due to the adverse effect of GnRH analogs and the negative effect of the aspiration of granulosa cells during the oocyte retrieval, and the resultant corpus lustrum’s inability to produce sufficient progesterone. Finally, it may induce iatrogenic LPD,23 with decreased endometrial receptivity and implantation rate.7,22 Because keeping with the natural processes and strengthening them brings about efficient results on most occasions, the current investigation aimed at assisting implantation by modulating the deficient luteal phase and endometrial thickness. To the best of our knowledge, this treatment has not been reported in previous studies about ART and the treatment proposed in the current investigation is the original innovation of the authors. The cornerstone of the methodology of the current study is precise patient selection in terms of inclusion and exclusion criteria mentioned in the methods. Nevertheless, future trials of this innovative treatment for assisted reproduction of other patients, e.g. for aiding IVF in cases with RIF are, suggested.
The exact etiology of RIF, which is a rather prevalent clinical problem, is unknown and there is no unique treatment protocol. Therefore, many clinicians incorporate empirical approaches for the treatment of this infertility.24
Previous literature is the host of controversies concerning the effect of endometrial thickness on pregnancy achievement after embryo transfer cycles of IVF. Although some authors stated no significant relationship between endometrial thickness and pregnancy achievement.25,26 Many authors demonstrate a close relationship in this regard.27-29 Recent studies have even suggested that there is an optimum range of endometrial thickness, needed to obtain a successful pregnancy; which is between 8 and 15 mm.30-32 One study indicated no association between EMT and ongoing pregnancy in women undergoing intrauterine insemination (IUI) with gonadotrophin treatment.33 Another study reported in cases of ovarian stimulation-IUI for unexplained infertility, higher live birth rates are observed with an increment of EMT; however, EMT is not significantly associated with live birth rates when adjusted for the type of ovarian stimulation treatment.34 Nonetheless, in an extensive literature search, we found no report about effective interventions fortifying the physiologic reproductive cycle by endometrial thickening for increasing the rate of natural implantation and pregnancy achievement.
In the present study, significant endometrial thickening was induced by MPA administration followed by successful natural pregnancies in most of the cases. Several studies have shown that MPA has no embryopathy risk and is a safe medication during pregnancy.12,31 In this study, pregnancies were followed up until delivery in terms of miscarriage, IUFD, gestational diabetes mellitus (GDM), preeclampsia, eclampsia, preterm labor, CS, NVD, and embryopathy. Except for three pregnancy losses and one case of GDM, no other maternal complications and no embryopathies before and after birth were found. Most pregnant women in the present study gave birth by elective CS. The patient’s preference for CS delivery is high in Iran.
Moreover, couples achieving pregnancy after several years of infertility treasure their babies very much and fear complications during delivery, which is why they prefer CS delivery.
In this interventional study, we carefully assessed women who encountered RIF following several in vitro fertilization treatment cycles six months before the study and conducted serum biomarkers (β-hCG, progesterone, and estradiol levels) measurements and ultrasonic assessment before recruitment and evaluated them (60 eligible women) for six cycles. This process lasted more than two years. The most important limitation of the current study was patient selection due to narrow inclusion criteria.
Clinical implication
Using MPA in women with RIF following several in vitro fertilization due to unexplained infertility could be helpful in strengthening the conception results; however, the precise selection of cases is essential for this purpose.
Study Highlights
What is current knowledge?
What is new here?
Conclusion
The results of this study showed that oral prescription of MPA could be a promising approach for unexplained infertility complications due to RIF. This treatment led to pregnancy achievement by natural intercourse and did not induce any serious side effects.
Acknowledgments
Hereby, we appreciate all women who patiently participated in this study.
Competing Interests
The authors have no conflict of interest.
Ethical Approval
The project was approved by the Regional Ethics Committee of Tabriz University of Medical Sciences and the approval number is IR.TBZMED.REC.1395.1062. The procedures were in accordance with the Helsinki Declaration of 1975, as revised in 2008. Written informed consent to participate was obtained.
References
- Sadeghzadeh Oskouei B, Pashaiasl M, Heidari MH, Salehi M, Veladi H, Ghaderi Pakdel F. Evaluation of mouse oocyte in vitro maturation developmental competency in dynamic culture systems by design and construction of a lab on a chip device and its comparison with conventional culture system. Cell J 2016; 18(2):205-13. doi: 10.22074/cellj.2016.4315 [Crossref] [ Google Scholar]
- Farquhar C, Marjoribanks J. Assisted reproductive technology: an overview of Cochrane reviews. Cochrane Database Syst Rev 2018; 8(8):CD010537. doi: 10.1002/14651858.CD010537.pub5 [Crossref] [ Google Scholar]
- Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod 2006; 21(12):3036-43. doi: 10.1093/humrep/del305 [Crossref] [ Google Scholar]
- Ocal P, Cift T, Bulut B, Balcan E, Cepni I, Aydogan B. Recurrent implantation failure is more frequently seen in female patients with poor prognosis. Int J Fertil Steril 2012; 6(2):71-8. [ Google Scholar]
- van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2015; 2015(7):CD009154. doi: 10.1002/14651858.CD009154.pub3 [Crossref] [ Google Scholar]
- Erdem A, Erdem M, Atmaca S, Guler I. Impact of luteal phase support on pregnancy rates in intrauterine insemination cycles: a prospective randomized study. Fertil Steril 2009; 91(6):2508-13. doi: 10.1016/j.fertnstert.2008.04.029 [Crossref] [ Google Scholar]
- Lawlor DA, Nelson SM. Effect of age on decisions about the numbers of embryos to transfer in assisted conception: a prospective study. Lancet 2012; 379(9815):521-7. doi: 10.1016/s0140-6736(11)61267-1 [Crossref] [ Google Scholar]
- Griesinger G, Meldrum D. Introduction: management of the luteal phase in assisted reproductive technology. Fertil Steril 2018; 109(5):747-8. doi: 10.1016/j.fertnstert.2018.02.009 [Crossref] [ Google Scholar]
- Günther V, Otte SV, Freytag D, Maass N, Alkatout I. Recurrent implantation failure–an overview of current research. Gynecol Endocrinol 2021; 37(7):584-90. doi: 10.1080/09513590.2021.1878136 [Crossref] [ Google Scholar]
- Fatemi HM. The luteal phase after 3 decades of IVF: what do we know?. Reprod Biomed Online 2009; 19 Suppl 4:4331. [ Google Scholar]
- Daya S, Gunby J. Luteal phase support in assisted reproduction cycles. Cochrane Database Syst Rev. 2004(3):CD004830. 10.1002/14651858.cd004830.
- World Health Organization (WHO). WHO Laboratory Manual for the Examination and Processing of Human Semen. 6th ed. WHO; 2021. Available from: https://www.who.int/publications/i/item/9789240030787.
- Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod 2018; 33(10):1883-8. doi: 10.1093/humrep/dey281 [Crossref] [ Google Scholar]
- Quaas A, Dokras A. Diagnosis and treatment of unexplained infertility. Rev Obstet Gynecol 2008; 1(2):69-76. [ Google Scholar]
- Ebrahimi M, Akbari Asbagh F, Darvish S. The effect of luteal phase support on pregnancy rates of the stimulated intrauterine insemination cycles in couples with unexplained infertility. Int J Fertil Steril 2010; 4(2):51-6. doi: 10.22074/ijfs.2010.45823 [Crossref] [ Google Scholar]
- Czyzyk A, Podfigurna A, Genazzani AR, Meczekalski B. The role of progesterone therapy in early pregnancy: from physiological role to therapeutic utility. Gynecol Endocrinol 2017; 33(6):421-4. doi: 10.1080/09513590.2017.1291615 [Crossref] [ Google Scholar]
- Haas DM, Hathaway TJ, Ramsey PS. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database Syst Rev 2019; 2019(11):CD003511. doi: 10.1002/14651858.CD003511.pub5 [Crossref] [ Google Scholar]
- Piccinni MP, Lombardelli L, Logiodice F, Kullolli O, Maggi E, Barkley MS. Medroxyprogesterone acetate decreases Th1, Th17, and increases Th22 responses via AHR signaling which could affect susceptibility to infections and inflammatory disease. Front Immunol 2019; 10:642. doi: 10.3389/fimmu.2019.00642 [Crossref] [ Google Scholar]
- Rai R, Regan L. Recurrent miscarriage. Lancet 2006; 368(9535):601-11. doi: 10.1016/s0140-6736(06)69204-0 [Crossref] [ Google Scholar]
- Yovich JL, Turner SR, Draper R. Medroxyprogesterone acetate therapy in early pregnancy has no apparent fetal effects. Teratology 1988; 38(2):135-44. doi: 10.1002/tera.1420380206 [Crossref] [ Google Scholar]
- Bulletti C, Bulletti FM, Sciorio R, Guido M. Progesterone: the key factor of the beginning of life. Int J Mol Sci 2022; 23(22):14138. doi: 10.3390/ijms232214138 [Crossref] [ Google Scholar]
- Dashti S, Eftekhar M. Luteal-phase support in assisted reproductive technology: an ongoing challenge. Int J Reprod Biomed 2021; 19(9):761-72. doi: 10.18502/ijrm.v19i9.9708 [Crossref] [ Google Scholar]
- Sullivan DH. What to do when you can’t get pregnant: the complete guide to all the options for couples facing fertility issues. International Journal of Childbirth Education 2014; 29(2):94-6. [ Google Scholar]
- Moustafa S, Young SL. Diagnostic and therapeutic options in recurrent implantation failure. F1000Res 2020; 9:F1000 Faculty Rev-208. doi: 10.12688/f1000research.22403.1 [Crossref] [ Google Scholar]
- Liu Y, Ye XY, Chan C. The association between endometrial thickness and pregnancy outcome in gonadotropin-stimulated intrauterine insemination cycles. Reprod Biol Endocrinol 2019; 17(1):14. doi: 10.1186/s12958-019-0455-1 [Crossref] [ Google Scholar]
- Ribeiro VC, Santos-Ribeiro S, De Munck N, Drakopoulos P, Polyzos NP, Schutyser V. Should we continue to measure endometrial thickness in modern-day medicine? The effect on live birth rates and birth weight. Reprod Biomed Online 2018; 36(4):416-26. doi: 10.1016/j.rbmo.2017.12.016 [Crossref] [ Google Scholar]
- Shalom-Paz E, Atia N, Atzmon Y, Hallak M, Shrim A. The effect of endometrial thickness and pattern on the success of frozen embryo transfer cycles and gestational age accuracy. Gynecol Endocrinol 2021; 37(5):428-32. doi: 10.1080/09513590.2020.1821359 [Crossref] [ Google Scholar]
- De Geyter C, Schmitter M, De Geyter M, Nieschlag E, Holzgreve W, Schneider HP. Prospective evaluation of the ultrasound appearance of the endometrium in a cohort of 1,186 infertile women. Fertil Steril 2000; 73(1):106-13. doi: 10.1016/s0015-0282(99)00484-7 [Crossref] [ Google Scholar]
- Puerto B, Creus M, Carmona F, Civico S, Vanrell JA, Balasch J. Ultrasonography as a predictor of embryo implantation after in vitro fertilization: a controlled study. Fertil Steril 2003; 79(4):1015-22. doi: 10.1016/s0015-0282(02)04854-9 [Crossref] [ Google Scholar]
- Malhotra N, Shah P, Kumar P, Acharya P, Panchal S, Malhotra J. Ultrasound in Obstetrics & Gynecology. JP Medical Ltd; 2014.
- Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H. Predictive factors for pregnancy after intrauterine insemination (IUI): an analysis of 1038 cycles and a review of the literature. Fertil Steril 2010; 93(1):79-88. doi: 10.1016/j.fertnstert.2008.09.058 [Crossref] [ Google Scholar]
- Kolibianakis EM, Zikopoulos KA, Fatemi HM, Osmanagaoglu K, Evenpoel J, Van Steirteghem A. Endometrial thickness cannot predict ongoing pregnancy achievement in cycles stimulated with clomiphene citrate for intrauterine insemination. Reprod Biomed Online 2004; 8(1):115-8. doi: 10.1016/s1472-6483(10)60505-6 [Crossref] [ Google Scholar]
- Weiss NS, van Vliet MN, Limpens J, Hompes PGA, Lambalk CB, Mochtar MH. Endometrial thickness in women undergoing IUI with ovarian stimulation How thick is too thin? A systematic review and meta-analysis. Hum Reprod 2017; 32(5):1009-18. doi: 10.1093/humrep/dex035 [Crossref] [ Google Scholar]
- Quaas AM, Gavrizi SZ, Peck JD, Diamond MP, Legro RS, Robinson RD. Endometrial thickness after ovarian stimulation with gonadotropin, clomiphene, or letrozole for unexplained infertility, and association with treatment outcomes. Fertil Steril 2021; 115(1):213-20. doi: 10.1016/j.fertnstert.2020.07.030 [Crossref] [ Google Scholar]